Articles
- Page Path
- HOME > Epidemiol Health > Volume 45; 2023 > Article
-
Original Article
Age at first childbirth and the risk of hypertriglyceridemia among Korean women -
Hye Rin Choi1,2
 , Hyeon Chang Kim3
, Hyeon Chang Kim3
-
Epidemiol Health 2022;45:e2023010.
DOI: https://doi.org/10.4178/epih.e2023010
Published online: December 29, 2022
1Center for Cohort Studies, Total Healthcare Center, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
2Institute of Medical Research, Sungkyunkwan University School of Medicine, Suwon, Korea
3Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, Korea
- Correspondence: Hyeon Chang Kim Department of Preventive Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea E-mail: hckim@yuhs.ac
© 2023, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
OBJECTIVES
- We aimed to investigate the association of age at first childbirth with the risk of hypertriglyceridemia among Korean women.
-
METHODS
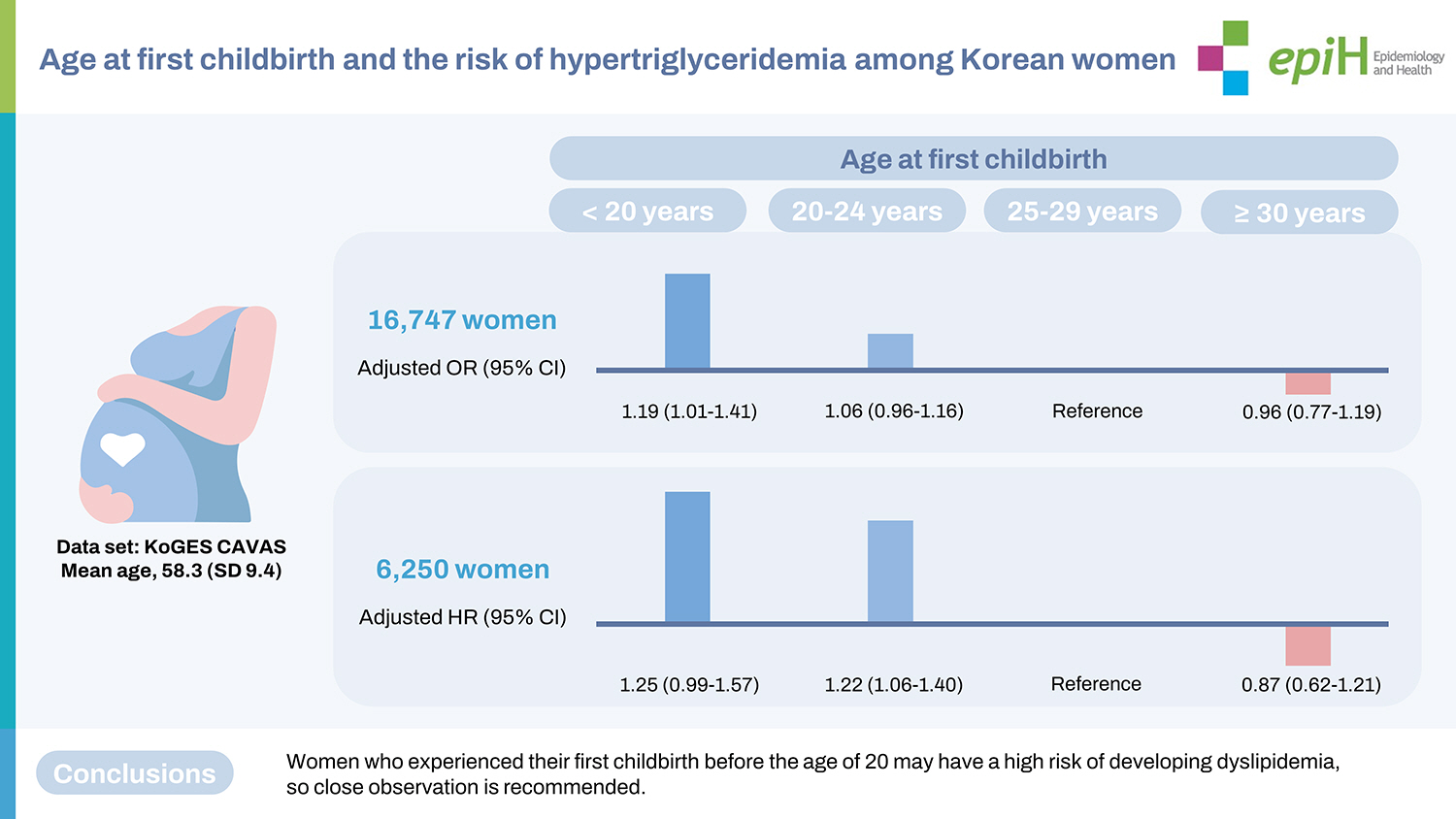
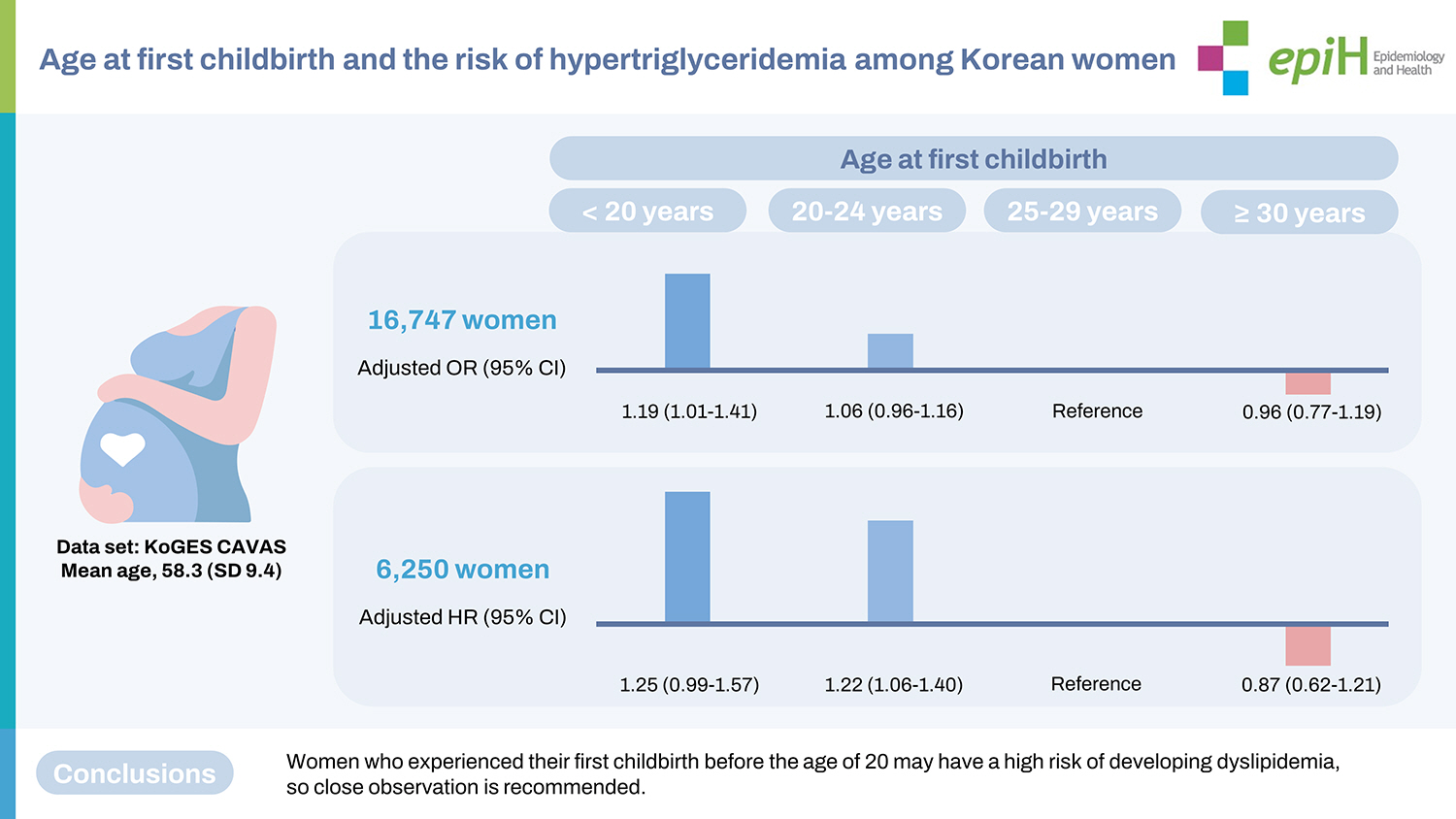
- This study used data from the Korean Genome and Epidemiology Study–Cardiovascular Disease Association Study. In total, 16,747 women were included in the cross-sectional analysis, and 6,250 women were included in the longitudinal analysis. The participants were divided based on their age at first childbirth (<20, 20-24, 25-29, and ≥30 years). Hypertriglyceridemia was defined as triglyceride levels of ≥150 mg/dL.
-
RESULTS
- The multivariable-adjusted odds ratio for prevalent hypertriglyceridemia was 1.19 (95% confidence interval [CI], 1.01 to 1.40) in women whose first childbirth was before 20 years of age, compared to those whose first childbirth was at 25-29 years of age, after adjustment for age, study site, body mass index, blood pressure, diabetes, alcohol consumption, carbohydrate intake, income, marital status, education, parity, usage of oral contraceptives, and hormone replacement status. During a median follow-up of 5.2 years, 1,770 women developed hypertriglyceridemia. Compared with women who gave birth to their first child between 25 years and 29 years of age, those giving birth to their first child before 20 years of age had a higher risk for incident hypertriglyceridemia in later life (adjusted hazard ratio, 1.25; 95% CI, 0.99 to 1.57).
-
CONCLUSIONS
- Giving birth to one’s first child before the age of 20 years was associated with an increased risk of hypertriglyceridemia among Korean women.
- Pregnancy and childbirth are major life events affecting metabolic function and the cardiovascular system [1,2]. Blood lipid profiles constantly change during menstruation, pregnancy, childbirth, and menopause [3-5]. In particular, serum triglyceride levels have been reported to predict maternal or fetal adverse health effects [6-9]. Estrogen also regulates lipoprotein metabolism. Increased estrogen levels reduce lipoprotein lipase secretion, thus elevating blood triglyceride levels [10,11]. As estrogen could cause severe hypertriglyceridemia [12,13], some researchers have suggested that clinicians should check blood triglyceride levels in reproductive-age women before initiating estrogen therapy.
- First childbirth before the age of 20 years has been linked to an increase in adverse cardiovascular health outcomes and metabolic disorders [14]. Pregnancy and childbirth in adolescence have been linked to a high risk of hypertriglyceridemia because changes in the lipid profile during pregnancy are consistent with the hormonal changes associated with the onset of puberty [15-17]. Most previous studies have investigated whether early menarche [18], early natural menopause [4,19], or abortions [20] were associated with metabolic syndrome [21]. However, these studies did not focus on the age at first childbirth. Furthermore, in the 1960s in Korea, women living in rural areas often got married early. Hence, they gave birth at an early age [22]. In this study, we targeted inhabitants of rural areas with a relatively diverse childbirth history to identify factors that might be associated with hypertriglyceridemia in later life. Therefore, this research evaluated whether the age at first childbirth was associated with adverse triglyceride levels in Korean women living in rural communities.
INTRODUCTION
- Study population
- This is a rural community-based cohort study that used data from the Korean Genome and Epidemiology Study–Cardiovascular Disease Association Study, which investigated the risk factors for cardiovascular disease (CVD) development [23]. Study participants aged ≥ 40 years at baseline were recruited between 2005 and 2011 from 11 rural communities in Korea. Follow-up examinations were conducted 4 times until 2016, after the baseline study. The mean follow-up duration was 5.2 years. In total, 28,337 participants (10,821 men and 17,516 women) completed the health examinations and questionnaires at baseline.
- Nulliparous women (n= 336) and women not having information about age at first childbirth (n= 414) or triglyceride levels (n= 19) were excluded from this cohort study. Finally, 16,747 women were included for a cross-sectional analysis. We included only women without hypertriglyceridemia at baseline for the longitudinal analysis to determine whether childbirth factors could affect newer-onset hypertriglyceridemia. Women who participated in at least 1 follow-up examination were included. Therefore, a total of 6,250 women were included in the investigation on longitudinal associations (Figure 1).
- Measurements
- Based on a predefined protocol, all participants were individually interviewed using standardized questionnaires by trained interviewers. The reproductive factors that were included were age at first childbirth, parity, age at menarche, menopausal status, usage of oral contraceptives, and hormone replacement status. The women were asked questions about their age at first childbirth, such as “Have you experienced pregnancy and delivery; if yes, then what was your age at first childbirth?”. Based on their age at first childbirth, we divided the study participants into 4 groups as follows: <20 years, 20-24 years, 25-29 years (reference), and ≥30 years. A history of health-related behaviors, including alcohol consumption, smoking status, and physical activity was taken. The participants were categorized as current drinkers or former/never drinkers based on their alcohol consumption. The participants were dichotomized by smoking status into current smokers or former/never smokers. However, the proportion of current smokers was very low; hence, we did not include smoking status in the analysis of this study. Physical activity was indicated by “yes” for participants who reported exercising enough to sweat at least once a week and “no” for those who did not. The socioeconomic status included household income, occupation, education, and marital status. Household income was defined as the average income of the household members per month and was divided into two groups: < 1,000 US dollar (USD) per month and ≥ 1,000 USD per month. The occupation was categorized as white, pink, or blue collar and others (housewives, students, and unemployed). Education levels were also classified into 2 levels: participants whose education was an elementary school level or lower and those who had an education level higher than elementary school. Marital status was dichotomized as living with a spouse or living alone (i.e., single, separated, divorced, and widowed). Participants wore lightweight clothing and no shoes for convenient and reliable examinations. The standing height was measured to the nearest 0.1 cm on a stadiometer, and body weight was measured to the nearest 0.1 kg on a digital scale. Body mass index (BMI) was calculated by dividing the body weight in kilograms by the height in meters squared (kg/m2). Blood pressure was recorded after participants rested for more than 5 minutes. Systolic and diastolic blood pressure were measured twice with at least a 5-minute interval between measurements using a standard mercury sphygmomanometer (Baumanometer; WA Baum, Copiague, NY, USA) and an automatic sphygmomanometer (Dinamap 1846 SX/P; GE Healthcare, Milwaukee, WI, USA) depending on the institution. Additional measurements were taken if the difference between the first and second measurements was greater than 10 mmHg. The average of the last 2 measurements was used for analysis. Hypertension was defined as a systolic blood pressure of ≥140 mmHg, a diastolic blood pressure of ≥ 90 mmHg, or the current use of blood pressure-lowering drugs.
- After at least 8 hours of fasting, blood samples of all participants were collected from the antecubital vein. Total cholesterol, high-density lipoprotein cholesterol, and triglyceride levels were determined using an enzymatic method (ADVIA 1800; Siemens Healthineers, Deerfield, IL, USA). Fasting glucose levels were assayed by a colorimetric method (ADVIA 1800; Siemens Healthineers), and fasting insulin levels were measured with an immunoradiometric assay (SR-300; Stratec, Birkenfeld, Germany). Hypertriglyceridemia was defined as a triglyceride level ≥ 150 mg/dL. Diabetes mellitus was defined as fasting glucose level ≥ 126 mg/dL or current treatment with oral antidiabetic agents or insulin.
- Statistical analysis
- Data were presented as mean values with standard deviations or numbers with percentages. For continuous variables with a skewed distribution, data were presented as median values with interquartile ranges. In order to investigate the linear trends of characteristics according to age at first childbirth, general linear models with contrast coefficients for linear trend tests for continuous variables were performed. The Cochran–Armitage test was also used for linear trends for categorical variables. Triglyceride values were log-transformed for parametric analysis because they were right-skewed. Multivariable logistic regression analysis was performed to examine the cross-sectional association between age at first childbirth and prevalent hypertriglyceridemia, using only baseline data. To adjust for potential confounders, model 1 was adjusted for age, study site, BMI, menopausal status (only for the total sample), blood pressure, and diabetes; model 2 was additionally adjusted for alcohol consumption, carbohydrate intake, income, marital status, and education; and model 3 was additionally adjusted for parity, usage of oral contraceptives and hormone replacement status. We selected the covariates from previous studies [14,24] and decided on the final adjusted model by considering the statistical implications. A sensitivity analysis was also conducted for women who had never taken lipid-lowering medication.
- Additionally, Cox proportional hazard models were conducted to determine whether the age at first childbirth affected the risk of new-onset hypertriglyceridemia in the follow-up period in both the total sample and postmenopausal women. In this analysis, we excluded participants who already had baseline hypertriglyceridemia or those who did not participate in any follow-up examinations. We defined the index date as the first health examination date when an individual’s serum triglyceride level was over 150 mg/dL. Person-years of follow-up were calculated from the initial examination to either the time of occurrence of hypertriglyceridemia or the date of the last health check-up, whichever came first. Hazard ratios (HRs) and 95% confidence interval (CIs) for developing hypertriglyceridemia during the follow-up period were estimated after adjusting for age, study site, BMI, menopausal status (only for the total sample), blood pressure, diabetes, alcohol consumption, carbohydrate intake, income, marital status, education, parity, usage of oral contraceptive pills, and hormone replacement status. We considered potential confounders that were measured at baseline visits. The proportional hazards assumption was checked before using the Cox proportional hazard regression model. The cumulative incidence of hypertriglyceridemia was drawn using Kaplan–Meier curves from the time-to-event analysis. Furthermore, the log-rank test was performed to compare the time to incident hypertriglyceridemia among the groups defined by age at first childbirth.
- All statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a 2-sided p-value of less than 0.05, and marginal significance was defined as a 2-sided p-value of less than 0.10.
- Ethics statement
- The study protocol was approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine (Y-2019-0170). All participants provided written informed consent.
MATERIALS AND METHODS
- Table 1 describes the baseline characteristics according to the age at first childbirth. Among 16,747 participants, 1,176 (7.0%) had their first childbirth at < 20 years, 9,283 (55.4%) from 20 to 24 years, 5,441 (32.5%) from 25 to 29 years, and 847 (5.1%) at ≥ 30 years. As the age at first childbirth increased, the mean age at baseline decreased significantly and the proportions of women with ≥ 5 children and blue-collar workers decreased significantly. Furthermore, BMI, waist circumference, systolic and diastolic blood pressure, fasting glucose and insulin, total cholesterol, and triglyceride levels decreased significantly as the age at first childbirth increased. Younger age at first childbirth was associated with a lower proportion of women living with their spouses and higher proportions of women with lower education levels and income less than 1,000 USD per month.
- Table 2 shows the cross-sectional associations between the age at first childbirth and prevalent hypertriglyceridemia at baseline among all participants and postmenopausal women. Overall, 41.6% of all women and 43.4% of postmenopausal women who had their first childbirth before the age of 20 had hypertriglyceridemia at the baseline examination. In the unadjusted model, the odds ratios (ORs) of prevalent hypertriglyceridemia were 1.78 (95% CI, 1.56 to 2.02) for women with their first childbirth at < 20 years and 1.35 (95% CI, 1.25 to 1.45) for women with their first childbirth between 20 years and 24 years, compared with those with their first birth at age 25-29. The negative associations between age at first childbirth and prevalent hypertriglyceridemia were significant (p < 0.001). After adjusting for age, study site, BMI, menopausal status (only for the total sample), blood pressure, diabetes, alcohol consumption, carbohydrate intake, income, marital status, education, parity, usage of oral contraceptives, and hormone replacement status, the association remained significant. Compared to women with their first childbirth between 25 years and 59 years old, the fully adjusted OR was 1.19 (95% CI, 1.00 to 1.40) for prevalent hypertriglyceridemia in the group with their first birth before age 20. When analyzed as a continuous variable, the OR of prevalent hypertriglyceridemia corresponding to a one-year decrease in the age of first childbirth was 1.01 (95% CI, 1.00 to 1.03). In the sensitivity analysis, we only included postmenopausal women. The results were similar to the main findings. The fully adjusted ORs for having hypertriglyceridemia at baseline were 1.19 (95% CI, 1.01 to 1.40) for women with their first childbirth at < 20 years and 1.06 (95% CI, 0.96 to 1.17) for those with their first childbirth at 20-24 years, compared to the group with their first childbirth at age 25-59. As shown in Supplementary Material 1, the age at first childbirth was inversely and significantly correlated with log-transformed triglyceride levels regardless of age adjustment. Furthermore, after excluding women who took lipidlowering medications, the associations between the age at first childbirth and prevalent hypertriglyceridemia remained significant (Supplementary Material 2). Women who experienced their first childbirth before 20 years had a higher OR (1.20; 95% CI, 1.01 to 1.42) for having hypertriglyceridemia than the reference group.
- Table 3 shows the longitudinal associations between age at first childbirth and the incidence of hypertriglyceridemia at follow-up visits in all participants and postmenopausal women. Supplementary Material 3 shows a comparison of baseline characteristics between respondents and non-respondents to follow-up examinations. In all women, the fully adjusted hazard ratios (HRs) for incident hypertriglyceridemia were 1.25 (95% CI, 0.99 to 1.57) and 1.22 (95% CI, 1.06 to 1.40) for women who had their first childbirth at < 20 years and 20-24 years, respectively, compared with the group with their first birth at 25-29 years old. In a sensitivity analysis, postmenopausal women with their first childbirth at < 20 years and 20-24 years had significantly higher HRs (1.25; 95% CI, 0.99 to 1.57 and 1.22; 95% CI, 1.06 to 1.40) for developing hypertriglyceridemia during the follow-up period than those with their first childbirth between 25 years and 29 years, after adjusting for age at baseline, study site, BMI, blood pressure, diabetes, alcohol consumption, carbohydrate intake, socioeconomic status, parity, usage of oral contraceptives, and hormone replacement status. Furthermore, when we restricted the participants to those who underwent at least 3 follow-up health examinations as a sensitivity analysis, the adjusted HRs for incident hypertriglyceridemia were 1.69 (95% CI, 1.11 to 2.58) and 1.35 (95% CI, 1.05 to 1.74) in those with first childbirth at < 20 years and 20-24 years, respectively, compared to the reference group (Supplementary Material 4).
- The cumulative incidence of hypertriglyceridemia based on age at first childbirth using Kaplan–Meier plots in the total sample and postmenopausal women is displayed in Figure 2. In women with their first childbirth at < 20 years, the onset of hypertriglyceridemia was earlier than in women who were older at their first childbirth. The difference between the 2 groups of age at first childbirth was also significant (p < 0.001). Among postmenopausal women, new-onset hypertriglyceridemia occurred significantly earlier in those who were younger at their first childbirth (p<0.001). Women who had their first childbirth at ≥ 30 years of age were more likely to develop late-onset hypertriglyceridemia.
RESULTS
- This study examined whether an independent association of age at first childbirth with prevalent or incident hypertriglyceridemia was present among Korean women living in rural areas. We found that an early age at first childbirth (i.e., < 20 years) was significantly associated with a higher likelihood of prevalent hypertriglyceridemia at baseline compared to women whose first childbirth occurred between 25 years and 29 years, in both the total sample and postmenopausal women. During the follow-up period, women with their first childbirth at < 20 years had a 26% higher risk of incident hypertriglyceridemia than those with their first childbirth at 25-29 years. Among postmenopausal women, the risk of hypertriglyceridemia in later life increased by about 3% as the age of first childbirth decreased by 1 year. Furthermore, in both total and postmenopausal women, early first childbirth hastened the development of hypertriglyceridemia.
- Our results were consistent with the results of previous studies [14,15,24-27]. A cross-sectional study of 4,262 postmenopausal women found that early pregnancy (maternal age at first delivery ≤ 20 years) was significantly associated with an increased risk of metabolic syndrome. Among the determinants of metabolic syndrome, the odds of having high triglyceride levels were 33.3% higher in women experiencing their first delivery at ≤ 20 years than in those experiencing their first delivery at ≥ 26 years [14]. A prospective cohort study in the United States with a 10-year follow-up suggested that adolescent pregnancy had atherogenic effects on blood lipids independent of weight gain after childbirth. Atherogenic lipid profiles from adolescence to adulthood are important risk factors for CVD events. Therefore, in women experiencing their first childbirth at ≤ 20 years, the lipid profile should be carefully monitored [15]. In a preliminary study using data from the International Mobility in Aging study, the authors found that adolescent childbirth was significantly associated with a high risk of CVD. As age at first childbirth increased, the Framingham risk scores decreased. Early age of first childbirth was associated with significantly higher Framingham risk scores for CVD than in nulliparous women [24]. A review suggested that an early age at first childbirth resulted in adverse physiological and sociological outcomes throughout the lifespan. In women with their first childbirth before 20 years of age, the risk of overall CVD events increased [25]. Both previous papers observed an association between age at first childbirth and CVD outcomes. Since the prevalence of hypertriglyceridemia is a major risk factor for CVD, those results could support our findings about the association between the age at first childbirth and maternal hypertriglyceridemia in later life. Furthermore, women with their first childbirth before 20 years of age had a higher likelihood of developing elevated triglyceride levels even postpartum because pregnant teenagers might be exposed to higher risks of adverse physical changes than adult pregnant women. Pregnancy-related changes contributed to longer exposure to CVD risk factors, including hypertriglyceridemia, than in women with their first childbirth in adulthood [24]. An earlier age at first birth was significantly associated with a higher BMI, body weight, and waist circumference [14]. Obesity induced by childbirth might have a significant relationship with hypertriglyceridemia in later life. Even though previous studies have investigated the biological mechanisms between adolescent childbirth and hypertriglyceridemia, the exact mechanism has not been identified.
- The current study has several limitations. First, there might have been recall bias about participants’ reproductive history, which was collected by self-reported questionnaires. Moreover, women’s reproductive information might not have been accurate because visiting an obstetrics and gynecology clinic was less commonplace when many of the participants were pregnant. Second, as hypertriglyceridemia could be confirmed only based on an assessment of blood triglyceride levels, misclassification of hypertriglyceridemia cases could have taken place. Third, the diagnosis date of incident hypertriglyceridemia could not be identified. However, we found that young age at first childbirth might be associated with an increased risk of incident hypertriglyceridemia over time. Thus, the results of the longitudinal analysis provided additional evidence for the causal association of first childbirth with hypertriglyceridemia in later life. Fourth, although we considered several potential confounders, it is possible that unmeasured factors, including reproductive hormones (e.g., estrogen or follicle-stimulating hormone), could have affected the association of first childbirth with incident hypertriglyceridemia. As with the use of reproductive hormones, further longitudinal research could be conducted to assess whether changes in estrogen levels according to childbirth-related characteristics are associated with the development of hypertriglyceridemia. Lastly, this study was conducted among Korean women living in rural communities. Therefore, it is difficult to generalize the results to other ethnic populations.
- This study found that giving birth to one’s first child before 20 years of age might lead to high triglyceride levels, resulting in cardiovascular health problems in later life. Reproductive history was found to be independently predictive of CVD and might be useful for CVD prevention efforts. In women whose first childbirth occurred before 20 years of age, targeted preventive actions should be developed to manage lipid profiles. Further studies are needed to investigate the underlying mechanisms.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Material 1.
Supplementary Material 2
Supplementary Material 3
Supplementary Material 4
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study
-
FUNDING
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2004-E71004-00, 2005-E71011-00, 2006-E71009-00, 2007-E71002-00, 2008-E71004-00, 2009-E71006-00, 2010-E71003-00, 2011-E71002-00, 2012-E71007-00, 2013-E71008-00, 2014-E71006-00, 2014-E71006-01, 2016-E71001-00, 2017N-E71001-00) and the Health Fellowship Foundation.
-
AUTHOR CONTRIBUTIONS
Both authors contributed equally to conceiving the study, analyzing the data, and writing this paper.
NOTES
ACKNOWLEDGEMENTS

| Age at first childbirth | No. of women | Case, n (%)1 |
OR (95% CI) for hypertriglyceridemia |
|||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 12 | Model 23 | Model 34 | |||||
| Total women | ||||||||
| Continuous, per 1 yr earlier | 16,747 | 5,515 (32.9) | 1.06 (1.05, 1.07) | 1.02 (1.01, 1.03) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.03) | ||
| Category | ||||||||
| <20 | 1,176 | 489 (41.6) | 1.78 (1.56, 2.02) | 1.17 (1.02, 1.35) | 1.11 (0.97, 1.29) | 1.19 (1.01, 1.40) | ||
| 20-24 | 9,283 | 3,253 (35.0) | 1.35 (1.25, 1.45) | 1.08 (0.99, 1.16) | 1.03 (0.95, 1.11) | 1.06 (0.96, 1.16) | ||
| 25-29 | 5,441 | 1,557 (28.6) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||
| ≥30 | 847 | 216 (25.5) | 0.85 (0.72, 1.01) | 0.91 (0.76, 1.07) | 0.95 (0.80, 1.13) | 0.96 (0.77, 1.19) | ||
| p for trend | <0.001 | 0.003 | 0.128 | <0.001 | ||||
| Postmenopausal women | ||||||||
| Continuous, per 1 yr earlier | 12,931 | 4,667 (36.1) | 1.05 (1.04, 1.06) | 1.03 (1.01, 1.04) | 1.02 (0.01, 1.03) | 1.01 (1.00, 1.03) | ||
| Category | ||||||||
| <20 | 1,053 | 457 (43.4) | 1.62 (1.41, 1.86) | 1.27 (1.10, 1.48) | 1.24 (1.07, 1.44) | 1.19 (1.01, 1.40) | ||
| 20-24 | 7,666 | 2,867 (37.4) | 1.26 (1.16, 1.37) | 1.12 (1.03, 1.23) | 1.09 (1.00, 1.20) | 1.06 (0.96, 1.17) | ||
| 25-29 | 3,705 | 1,192 (32.2) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||
| ≥30 | 507 | 151 (29.8) | 0.89 (0.73, 1.10) | 0.90 (0.73, 1.11) | 0.93 (0.76, 1.15) | 0.96 (0.77, 1.19) | ||
| p for trend | <0.001 | <0.001 | 0.002 | <0.001 | ||||
OR, odds ratio; CI, confidence interval.
1 Cases for hypertriglyceridemia.
2 Model 1: adjusted for age, study site, body mass index, menopausal status (only for the total sample), blood pressure, and diabetes.
3 Model 2: adjusted for age, study site, body mass index, menopausal status (only for the total sample), blood pressure, diabetes, alcohol consumption, carbohydrate intake, income, marital status, and education.
4 Model 3: adjusted for age, study site,, body mass index, menopausal status (only for the total sample), blood pressure, diabetes, alcohol consumption, carbohydrate intake, income, marital status, education, parity, usage of oral contraceptives, and hormone replacement status.
| Age at first childbirth | No. at risk | Events1 | PY | Incidence rate (/100 PY) |
HR (95% CI) for hypertriglyceridemia |
||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 12 | Model 23 | |||||||
| Total women | |||||||||
| Continuous, per 1 yr earlier | 6,250 | 1,770 | 32,360 | 5.47 | 1.04 (1.02, 1.05) | 1.02 (1.00, 1.03) | 1.02 (1.00, 1.04) | ||
| Category | |||||||||
| <20 | 440 | 142 | 2,096.2 | 6.77 | 1.44 (1.20, 1.74) | 1.22 (1.00, 1.49) | 1.25 (0.99, 1.57) | ||
| 20-24 | 3,350 | 1,012 | 17,332.2 | 5.84 | 1.23 (1.11, 1.37) | 1.15 (1.03, 1.29) | 1.22 (1.06, 1.40) | ||
| 25-29 | 2,120 | 532 | 11,189.2 | 4.75 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||
| ≥30 | 340 | 84 | 1,742.4 | 4.82 | 1.01 (0.81, 1.28) | 1.05 (0.83, 1.32) | 0.87 (0.62, 1.21) | ||
| p for trend | <0.001 | 0.023 | 0.001 | ||||||
| Postmenopausal women | |||||||||
| Continuous, per 1 yr earlier | 4,584 | 1,376 | 23,433.9 | 5.87 | 1.03 (1.02, 1.05) | 1.02 (1.00, 1.04) | 1.02 (1.00, 1.04) | ||
| Category | |||||||||
| <20 | 382 | 121 | 1,804.6 | 6.71 | 1.33 (1.08, 1.64) | 1.17 (0.94, 1.46) | 1.25 (0.99, 1.57) | ||
| 20-24 | 2,661 | 846 | 13,513.1 | 6.26 | 1.24 (1.09, 1.40) | 1.18 (1.03, 1.35) | 1.22 (1.06, 1.40) | ||
| 25-29 | 1,357 | 364 | 7,142 | 5.1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||
| ≥30 | 184 | 45 | 974.2 | 4.62 | 0.90 (0.66, 1.23) | 0.90 (0.66, 1.22) | 0.87 (0.62, 1.21) | ||
| p for trend | <0.001 | 0.023 | 0.001 | ||||||
PY, person-years; HR, hazard ratio; CI, confidence interval.
1 Hypertriglyceridemia events.
2 Model 1: adjusted for age, study site, body mass index, menopausal status (only for the total sample), blood pressure, diabetes, alcohol consumption, carbohydrate intake, income, marital status, and education;
3 Model 2: adjusted for age, study site, body mass index, menopausal status (only for the total sample), blood pressure, diabetes, alcohol consumption, carbohydrate intake, income, marital status, education, parity, usage of oral contraceptives, and hormone replacement status.
- 1. Geraghty AA, Alberdi G, O’Sullivan EJ, O’Brien EC, Crosbie B, Twomey PJ, et al. Maternal and fetal blood lipid concentrations during pregnancy differ by maternal body mass index: findings from the ROLO study. BMC Pregnancy Childbirth 2017;17:360.ArticlePubMedPMCPDF
- 2. Bartels Ä, O’Donoghue K. Cholesterol in pregnancy: a review of knowns and unknowns. Obstet Med 2011;4:147-151.ArticlePubMedPMCPDF
- 3. Chiang AN, Yang ML, Hung JH, Chou P, Shyn SK, Ng HT. Alterations of serum lipid levels and their biological relevances during and after pregnancy. Life Sci 1995;56:2367-2375.ArticlePubMed
- 4. Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA 2019;322:2411-2421.ArticlePubMedPMC
- 5. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women’s Health Across the Nation. Am J Epidemiol 2009;169:1352-1361.ArticlePubMedPMC
- 6. Son GH, Kwon JY, Kim YH, Park YW. Maternal serum triglycerides as predictive factors for large-for-gestational age newborns in women with gestational diabetes mellitus. Acta Obstet Gynecol Scand 2010;89:700-704.ArticlePubMed
- 7. Hummel L, Schwartze A, Schirrmeister W, Wagner H. Maternal plasma triglycerides as a source of fetal fatty acids. Acta Biol Med Ger 1976;35:1635-1641.PubMed
- 8. Barrett HL, Dekker Nitert M, Jones L, O’Rourke P, Lust K, Gatford KL, et al. Determinants of maternal triglycerides in women with gestational diabetes mellitus in the Metformin in Gestational Diabetes (MiG) study. Diabetes Care 2013;36:1941-1946.ArticlePubMedPMCPDF
- 9. Hashemipour S, Haji Seidjavadi E, Maleki F, Esmailzadehha N, Movahed F, Yazdi Z. Level of maternal triglycerides is a predictor of fetal macrosomia in non-obese pregnant women with gestational diabetes mellitus. Pediatr Neonatol 2018;59:567-572.ArticlePubMed
- 10. Herrera E, Lasunción MA, Gomez-Coronado D, Aranda P, López-Luna P, Maier I. Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. Am J Obstet Gynecol 1988;158:1575-1583.ArticlePubMed
- 11. Baynes JW, Dominiczak MH. Medical biochemistry. 3rd ed. Mosby: Elsevier; 2009. p 385-405.
- 12. Goldenberg NM, Wang P, Glueck CJ. An observational study of severe hypertriglyceridemia, hypertriglyceridemic acute pancreatitis, and failure of triglyceride-lowering therapy when estrogens are given to women with and without familial hypertriglyceridemia. Clin Chim Acta 2003;332:11-19.ArticlePubMed
- 13. Glueck CJ, Lang J, Hamer T, Tracy T. Severe hypertriglyceridemia and pancreatitis when estrogen replacement therapy is given to hypertriglyceridemic women. J Lab Clin Med 1994;123:59-64.PubMed
- 14. Sim JH, Chung D, Lim JS, Lee MY, Chung CH, Shin JY, et al. Maternal age at first delivery is associated with the risk of metabolic syndrome in postmenopausal women: from 2008-2010 Korean National Health and Nutrition Examination Survey. PLoS One 2015;10:e0127860.ArticlePubMedPMC
- 15. Gunderson EP, Schreiber G, Striegel-Moore R, Hudes M, Daniels S, Biro FM, et al. Pregnancy during adolescence has lasting adverse effects on blood lipids: a 10-year longitudinal study of black and white females. J Clin Lipidol 2012;6:139-149.ArticlePubMed
- 16. Dai S, Fulton JE, Harrist RB, Grunbaum JA, Steffen LM, Labarthe DR. Blood lipids in children: age-related patterns and association with body-fat indices: project HeartBeat! Am J Prev Med 2009;37(1 Suppl):S56-S64.PubMed
- 17. Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, et al. Distributions and trends of serum lipid levels among United States children and adolescents ages 4-19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med 1998;27:879-890.ArticlePubMed
- 18. Feng Y, Hong X, Wilker E, Li Z, Zhang W, Jin D, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis 2008;196:590-597.ArticlePubMed
- 19. Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: long-term health consequences. Maturitas 2010;65:161-166.ArticlePubMed
- 20. Horn J, Tanz LJ, Stuart JJ, Markovitz AR, Skurnik G, Rimm EB, et al. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: a prospective cohort study. BJOG 2019;126:33-42.ArticlePDF
- 21. Vladutiu CJ, Siega-Riz AM, Sotres-Alvarez D, Stuebe AM, Ni A, Tabb KM, et al. Parity and components of the metabolic syndrome among US Hispanic/Latina women: results from the Hispanic Community Health Study/Study of Latinos. Circ Cardiovasc Qual Outcomes 2016;9(2 Suppl 1):S62-S69.PubMedPMC
- 22. Jun KH. Fertility transition in Korea: trend and prospect. J Korean Official Stat 2003;8:33-58 (Korean).
- 23. Kim Y, Han BG; KoGES group. Cohort profile: the Korean Genome and Epidemiology Study (KoGES) consortium. Int J Epidemiol 2017;46:e20.ArticlePubMed
- 24. Rosendaal NT, Alvarado B, Wu YY, Velez MP, da Câmara SM, Pirkle CM. Adolescent childbirth is associated with greater Framingham risk scores for cardiovascular disease among participants of the IMIAS (International Mobility in Aging Study). J Am Heart Assoc 2017;6:e007058.ArticlePubMedPMC
- 25. Rosendaal NT, Pirkle CM. Age at first birth and risk of later-life cardiovascular disease: a systematic review of the literature, its limitation, and recommendations for future research. BMC Public Health 2017;17:627.ArticlePubMedPMCPDF
- 26. Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith West D, Sidney S. Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults study. Am J Epidemiol 2004;159:1028-1039.ArticlePubMed
- 27. Cohen A, Pieper CF, Brown AJ, Bastian LA. Number of children and risk of metabolic syndrome in women. J Womens Health (Larchmt) 2006;15:763-773.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Adolescent childbirth and mobility disability among women ages 15–49: an analysis of population health surveys from 14 low-income and middle-income countries
Katherine E Peck, Diego G Bassani, Saionara MA Camara, Marlos R Domingues, Tetine Sentell, Maria P Velez, Catherine M Pirkle
BMJ Open.2023; 13(7): e072535. CrossRef

 KSE
KSE

 PubReader
PubReader ePub Link
ePub Link Cite
Cite