Articles
- Page Path
- HOME > Epidemiol Health > Volume 44; 2022 > Article
-
Original Article
Blood lipid levels and all-cause mortality in older adults: the Chinese Longitudinal Healthy Longevity Survey 2008-2018 -
Rongxi Wang*
 , Xiaoyue Yu*
, Xiaoyue Yu* , Zhiqiang Wang*
, Zhiqiang Wang* , Yujie Liu, Hui Chen, Shangbin Liu, Chen Xu, Yingjie Chen, Xin Ge, Danni Xia, Ruijie Chang, Gang Xu, Mi Xiang, Ying Wang, Tian Shen, Fan Hu
, Yujie Liu, Hui Chen, Shangbin Liu, Chen Xu, Yingjie Chen, Xin Ge, Danni Xia, Ruijie Chang, Gang Xu, Mi Xiang, Ying Wang, Tian Shen, Fan Hu , Yong Cai
, Yong Cai
-
Epidemiol Health 2022;44:e2022054.
DOI: https://doi.org/10.4178/epih.e2022054
Published online: July 5, 2022
School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- Correspondence: Yong Cai School of Public Health, Shanghai Jiao Tong University School of Medicine, South 227 Chongqing Road, Shanghai, China Email: caiyong202028@hotmail.com
- Co-correspondence: Fan Hu School of Public Health, Shanghai Jiao Tong University School of Medicine, South 227 Chongqing Road, Shanghai, China E-mail: hufan@sjtu.edu.cn
- *Wang, Yu & Wang contributed equally to this work as joint first authors.
©2022, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
OBJECTIVES
- Proper blood lipid levels are essential for survival in older adults, but inconsistent relationships have been reported between blood lipids and all-cause mortality in the elderly.
-
METHODS
- This retrospective longitudinal study analyzed data from 1,067 Chinese older adults enrolled in the Chinese Longitudinal Healthy Longevity Survey collected in 2008 and followed up until death or December 31, 2018. The outcome was all-cause mortality. Multivariate Cox regression analyses were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) with stratification by age (60-80, 80-100, or ≥100 years) for further analysis. The survival probability according to lipid profile quartiles was calculated using Kaplan-Meier curves and the log-rank test.
-
RESULTS
- The participants’ mean age was 84.84 years, and 57.0% were female. In total, 578 individuals died, and 277 were lost to follow-up. The mean total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels were higher among those who died than among those who survived. Participants in the second HDL-C quartile and the highest LDL-C and triglyceride (TG) quartiles had 28% higher, 23% lower, and 49% lower risks of all-cause mortality, respectively. After further adjustment, the associations remained except for HDL-C, and additional associations were observed between all-cause mortality and the third TC and LDL-C quartiles and the second TG quartile (HR, 1.44; 95% CI, 1.01 to 2.06; HR, 0.68; 95% CI, 0.49 to 0.94; HR, 0.79; 95% CI, 0.62 to 0.99, respectively).
-
CONCLUSIONS
- Older adults should maintain an LDL-C level of 1.91-2.47 mmol/L and a TG level of no less than 1.66 mmol/L.
- A consensus has been reached on the importance of maintaining low blood lipid levels to prevent cardiovascular disease due to the well-documented association between lipid levels and the risk of cardiovascular disease and death in middle-aged people. The survival advantage related to lower blood lipids may differ, however, for the elderly population. The U-shaped or inverse relationships found between total cholesterol (TC) levels and all-cause mortality in older people in Eastern and Western countries indicated that lower TC corresponded to a higher risk of death [1-5]. Several studies found low blood lipid levels to be associated with frailty and infectious diseases, and others identified an association with higher cancer mortality rates [6,7]. Furthermore, several studies have analyzed the proportions of different fractions of cholesterol such as low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG). Coronary heart disease (CHD) mortality has been found to have a positive relationship with LDL-C and TG and a negative relationship with HDL-C. However, studies on the relationship between all-cause mortality and lipid profiles have yielded inconsistent results, especially concerning the older population [8-13]. The general pattern that has emerged from prospective studies is that TC and LDL-C have a direct relationship with CHD mortality and an inverse association with all-cause mortality and mortality related to other chronic diseases, whereas HDL-C protects against both cardiovascular and non-cardiovascular disease mortality. However, whether this pattern corresponds to the elderly population in China remains unclear.
- In this study, baseline characteristics collected in 2008 and plasma biochemical parameters measured in 2009 from the fifth follow-up survey and survival information from the following 10 years from the Chinese Longitudinal Healthy Longevity Survey (CLHLS) were statistically analyzed to explore the associations between blood lipid fractions and all-cause mortality in elderly people in China and determine a reference lipid level for the improved survival of the elderly.
INTRODUCTION
- Study population
- The study population was selected from the CLHLS collected in 2008/2009 and 2018. The CLHLS is the first and largest nationwide, community-based, longitudinal prospective survey in China [14]. The CLHLS was designed to identify the determinants of the health and longevity of older adults. The CLHLS is conducted by randomly selecting participants from counties and cities across 22 provinces in China, covering about 85% of the total population of China [15]. The CLHLS was started in 1998, and follow-up surveys were conducted in 2000, 2002, 2005, 2008/2009, 2011/2012, 2014, and 2017/2018 [16]. The CLHLS contains a wide variety of information on demographics, lifestyle, diet, health status, and the daily activities of older people. In 2009, a biomarker sub-study was launched in 8 regions, including Sanshui in Guangdong Province, Yongfu in Guangxi Autonomous Area, Chengmai in Hainan Province, Xiayi in Henan Province, Zhongxiang in Hubei Province, Mayang in Hunan Province, Laizhou in Shandong Province, and Rudong in Jiangsu Province.
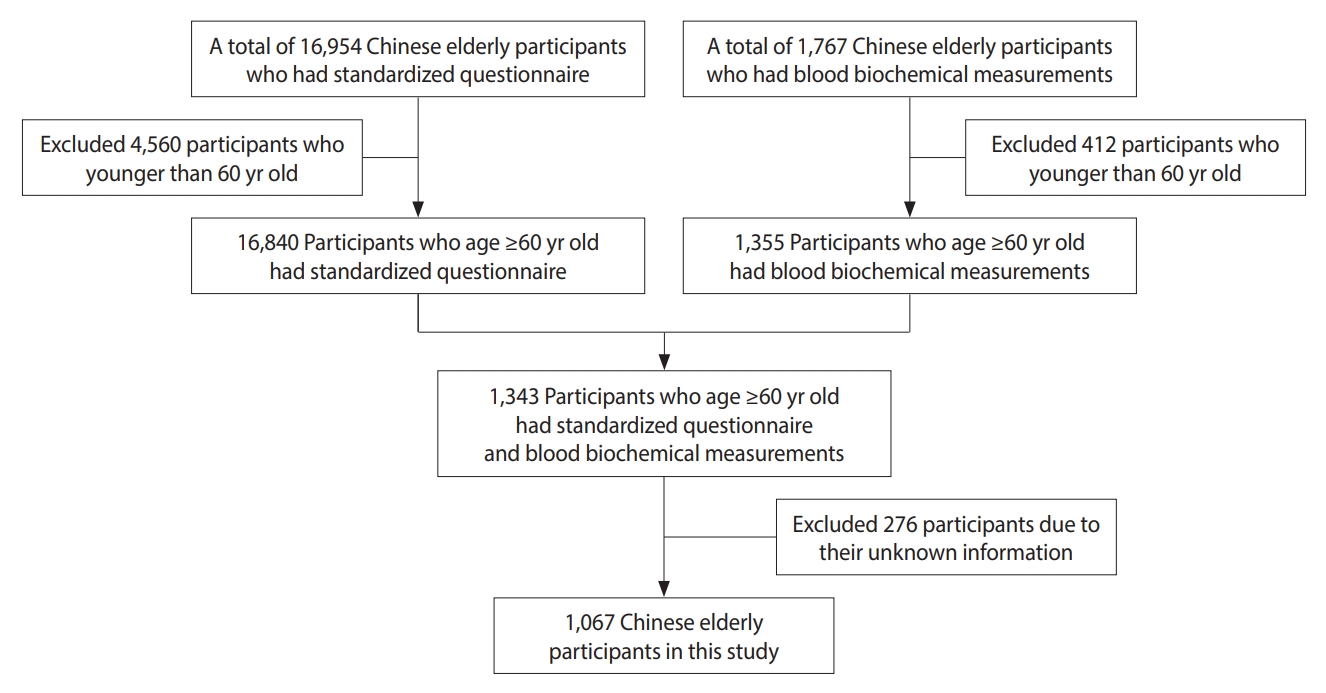
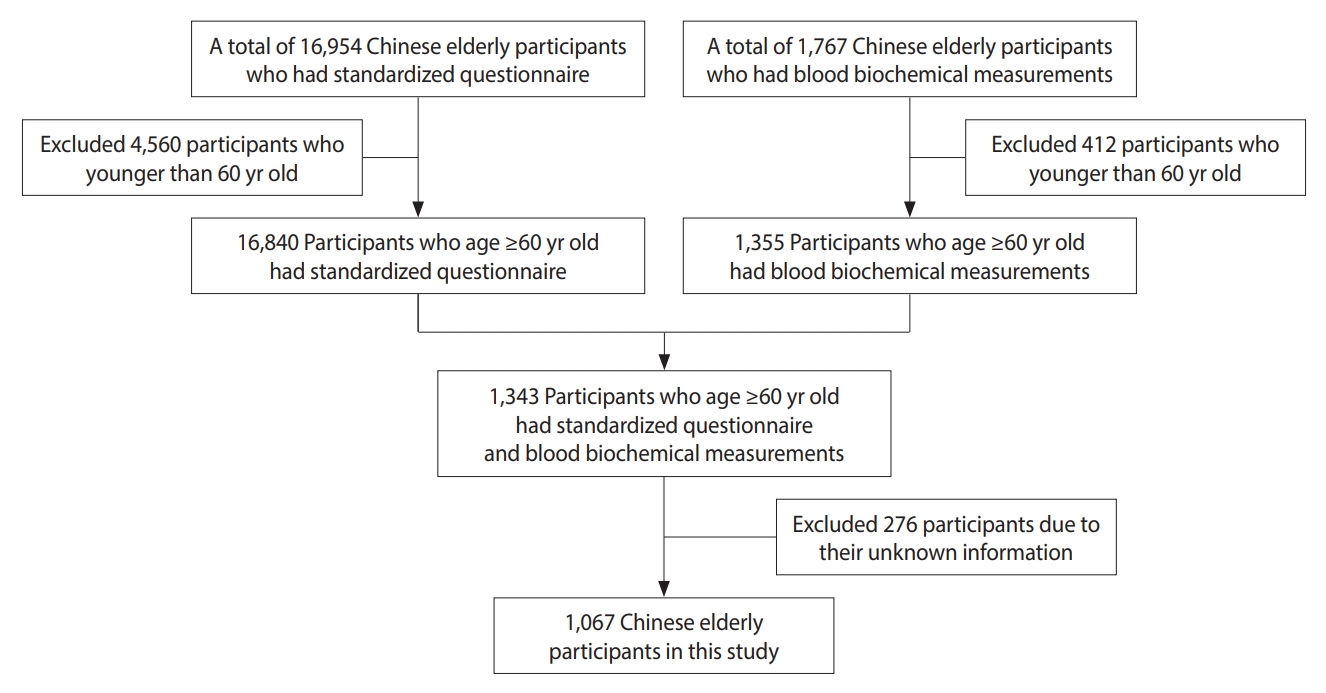
- We included 1,067 Chinese elderly participants in this study after applying the following exclusion criteria: (1) participants younger than 60 years of age and (2) participants with missing information. The flow chart of the study population is shown in Figure 1.
- Data collection
- We collected information using a standardized questionnaire administered through household interviews. The information included demographic data such as age, sex, residence, marital status, and economic status. After the household interview, each participant was asked to undergo anthropometric measurements, which included systolic blood pressure (SBP, mmHg), diastolic blood pressure (DBP, mmHg), and body mass index (BMI, kg/m2). Blood biochemistry tests were analyzed in this study, including blood urea nitrogen (mmol/L), plasma creatine (mmol/L), uric acid (μmol/L), plasma glucose (mmol/L), TC (mmol/L), HDL-C (mmol/L), LDL-C (mmol/L), and TG (mmol/L) levels.
- Biochemical measurements
- Fasting venous blood samples were collected by trained medical personnel from all willing participants after fasting overnight. Five milliliters of fasting venous blood were collected in heparin anticoagulant vacuum tubes and centrifuged at 20°C and 2,500 RPM for 10 minutes. The plasma was isolated and frozen at -20°C, shipped through a cold chain to the central laboratory at Capital Medical University in Beijing, and stored at -80°C until biochemical analysis. Blood urea nitrogen, plasma creatine, uric acid, plasma glucose, TC, HDL-C, LDL-C, and TG levels were measured using an automatic biochemical analyzer (Hitachi 7180; Hitachi High-Technologies, Tokyo, Japan).
- Study outcome during prospective follow-up
- The outcome of our study was all-cause mortality from January 1, 2008, to December 31, 2018. Mortality status was obtained from the publicly available CLHLS dataset, which contained data on the vital status of the survey participants from baseline to December 31, 2018. If a participant missed the follow-up visit, the survival time was defined as the interval between baseline and the time of the missed visit.
- Statistical analysis
- The study population was divided into 4 groups based on quartiles of lipid profiles. Continuous variables were presented as the mean and standard deviation (mean± SD) and compared using one -way analysis of variance. The categorical variables were presented in terms of numerical counts (%) and were compared using the chi-square test. Multivariate Cox regression analyses were used to investigate the relationships between quartiles of lipid profiles with all-cause mortality, and the results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Associations between quartiles of lipid profiles concerning all-cause mortality were assessed using 4 models as follows: quartiles for TC, age, sex, category of residence, marital status, and economic status were entered in model 1; model 2 was further adjusted for SBP, DBP, and BMI; model 3 was further adjusted for blood urea nitrogen, plasma creatine, uric acid, and plasma glucose levels; and quartiles for HDL-C, LDL-C, and TG levels were entered in model 4. A similar process was applied to quartiles of HDL-C, LDL-C, and TG. Age (60-80, 80-100, ≥ 100 years), sex (male, female), area of residence (city/town, rural), marital status (unmarried, married), economic status (< 10,000, ≥ 10,000 renminbi/yr), smoking status (no, yes) and drinking status (no, yes) were adjusted as categorical variables while SBP, DBP, BMI, blood urea nitrogen, plasma creatine, uric acid, and plasma glucose levels were adjusted as continuous variables. To confirm associations, sensitivity analysis was performed based on receiver operating characteristic (ROC) curves and the corresponding area under the curve (AUC). In addition, we performed subgroup analyses of the association between lipid profiles and all-cause mortality by age group (60-80, 80-100, or ≥ 100 years). Furthermore, the survival probability according to each lipid profile quartile was calculated using Kaplan-Meier curves, and the log-rank test was performed to analyze differences between quartiles. Statistical analysis was performed using the R version 4.03 (R Core Team, Vienna, Austria). A 2-sided p-value of < 0.05 was considered to indicate statistical significance.
- Ethics statement
- The CLHLS study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of Peking University (IRB00001052-13074). All participants provided written informed consent.
MATERIALS AND METHODS
- The sample consisted of 1,067 individuals aged 65-107 years old (mean±SD, 84.84±13.15), and the majority of participants were female (57.0%). The mean follow-up time was 62.97 months (range, 2-120). A total of 578 deaths (54.2%) were recorded, and 277 participants (26.0%) were lost to follow-up, the majority of whom were females. The mean TC, HDL-C, and LDL-C levels were higher in those who died than for those who survived, while TG levels were not (Table 1). The baseline characteristics of the study population according to the TC quartile are shown in Supplementary Material 1. The participants in the highest TC quartile were more likely to be between 80 years and 100 years of age, female, non-smokers, and non-drinkers as well as have a lower economic status, have higher levels of HDL-C and LDL-C, and reside in a rural area. Participants with the lowest concentrations of TC were more likely to have higher levels of plasma creatine and TG. The baseline characteristics according to blood lipid fraction quartile, including HDL-C, LDL-C, and TG, are shown in Supplementary Materials 1-4. Age-specific and sex-specific baseline characteristics are shown in Supplementary Material 5.
- All-cause mortality risk and lipid levels in different adjusted models
- As shown in Table 2, after adjustment for socio-demographic and lifestyle characteristics, participants in the second HDL-C quartile and the highest TG quartile had a 32% higher risk and 59% lower risk of all-cause mortality, respectively (HR, 1.32; 95% CI, 1.03 to 1.68; HR, 0.59; 95% CI, 0.46 to 0.77), compared to those in the lowest HDL-C and TG quartiles in model 1. After further adjustment in model 2 (based on model 1 with adjustments for SBP, DBP, and BMI), model 3 (based on model 2 with adjustments for blood urea nitrogen, plasma creatine, uric acid, and plasma glucose), and model 4 (based on model 3 with adjustments for TC, HDL-C, LDL-C, and TG), the association between TG and all-cause mortality remained significant in addition to the association between HDL-C and all-cause mortality other than that in model 4. In the fully adjusted model (model 4), however, an extra association between all-cause mortality and participants in the third TC and LDL-C quartiles and the second TG quartile appeared in comparison to other models (HR, 1.44; 95% CI, 1.01 to 2.06; HR, 0.68; 95% CI, 0.49 to 0.94; HR, 0.79; 95% CI, 0.62 to 0.99). Furthermore, the third LDL-C quartile protected against all-cause mortality in model 3 only (HR, 0.77; 95% CI, 0.61 to 0.99), and the association between LDL-C and all-cause mortality was not significant in the other models.
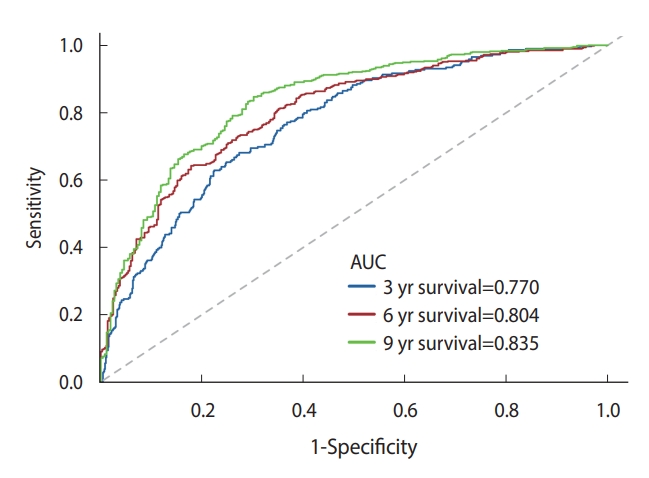
- Sensitivity analysis
- The multivariate model’s discriminative performance was assessed using ROC curves and the AUC. The AUCs for 3-year, 6-year, and 9-year all-cause mortality were 0.770, 0.804, and 0.835, respectively (Figure 2).
- Subgroup analysis
- Further exploration of the associations between TC (and its fractions) and all-cause mortality based on 3 different age groups is shown in Supplementary Materials 6-8. For participants between 60 years and 80 years of age, an increased risk of all-cause mortality was observed in all models for the second HDL-C quartile only. For participants between 80 years and 100 years of age, there was an increased risk of all-cause mortality for those in the third TC quartile, but a protective effect against all-cause mortality was observed in the highest TG quartile in all models. In addition, those in the second TC quartile had an increased risk of all-cause mortality (HR, 1.62; 95% CI, 1.08 to 2.43), while the second LDL-C quartile showed a protective effect against all-cause mortality in model 4 only (HR, 0.67; 95% CI, 0.47 to 0.95). For those over 100 years of age, none of the associations between TC quartiles (and the fractions of cholesterol) and all-cause mortality were statistically significant in any of the models.
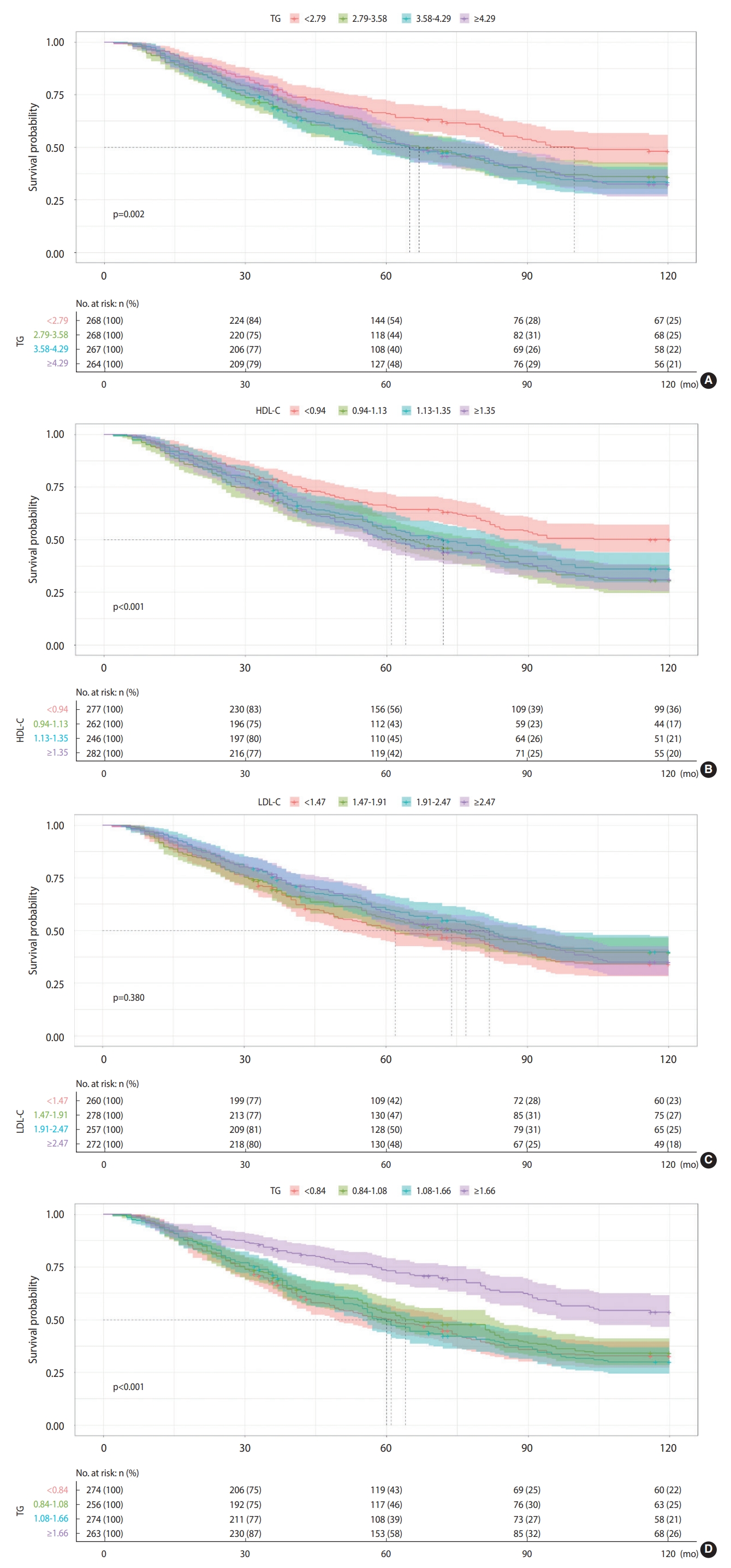
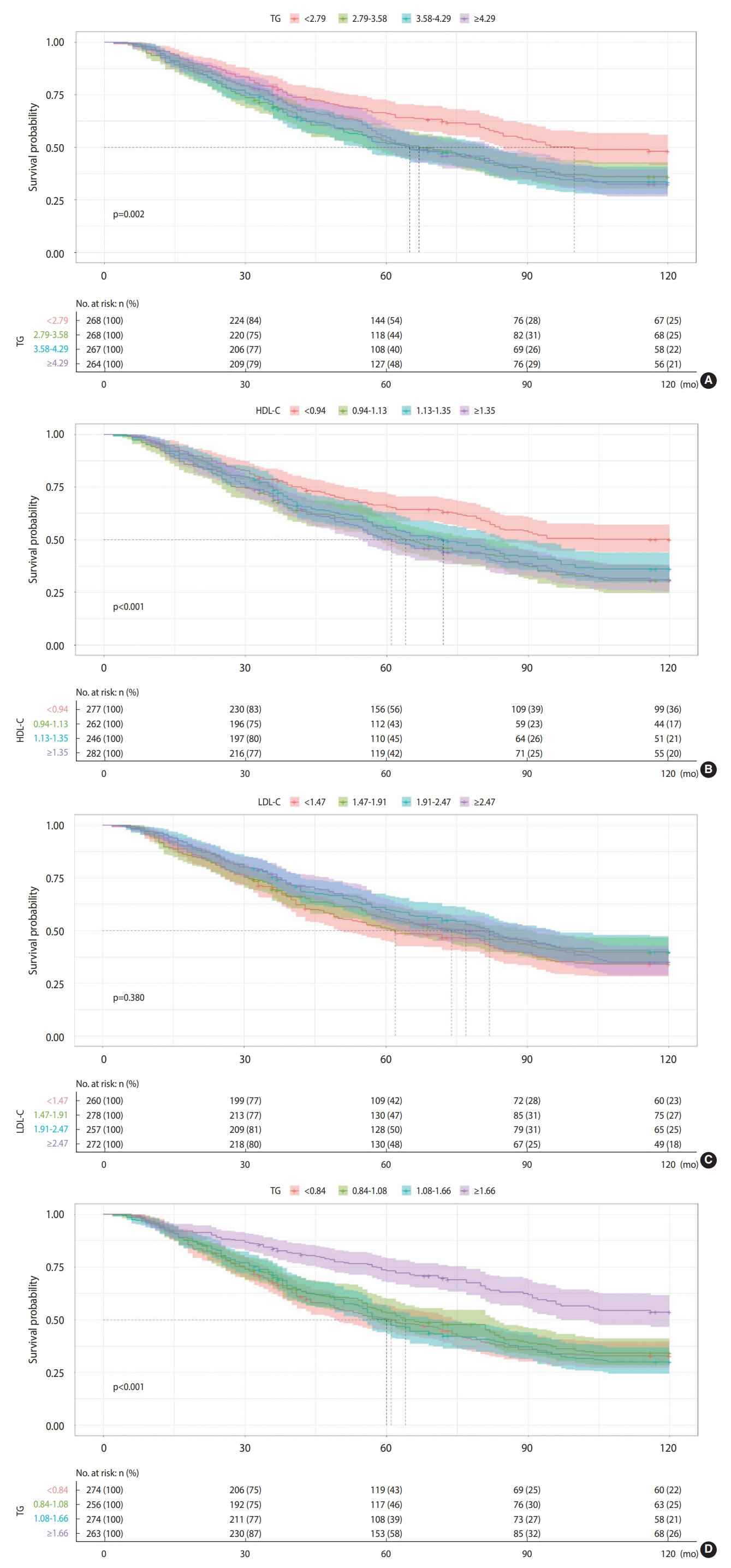
- Survival analysis
- Figure 3 shows the Kaplan-Meier survival curves for all-cause mortality in 4 different blood lipid groups (TC, HDL-C, LDL-C, and TG). For the relationship between TC, HDL-C, and all-cause mortality, participants in the lowest quartiles were more likely to have a significantly higher survival probability (p=0.002, < 0.001, respectively) than those in the higher quartiles, while participants in the highest TG quartile were more likely to have a significantly higher survival probability (p<0.001). The survival probability between participants in each of the 4 LDL-C quartiles, however, did not significantly differ (p=0.380).
RESULTS
- The present study found that higher LDL-C and TG levels were significantly associated with lower all-cause mortality, indicating better survival, while higher TC levels increased the risk of all-cause mortality and higher HDL-C levels did not increase the subsequent risk of mortality. The mortality risk of older adults decreased by 32% among those in the third LDL-C quartile (1.91-2.47 mmol/L) and by 45% among those in the fourth TG quartile (≥ 1.66 mmol/L). In addition, this trend was inconsistent with regard to each age group. Blood lipid levels may not affect the survival of those aged 100 years and older as much as it does those younger than 100 years of age.
- In the model that initially included quartiles of blood lipids, TC, HDL-C, and TG were meaningful. The second and third TC quartiles and second HDL-C quartile increased the risk of death, while the fourth TG quartile decreased all-cause mortality. This echoes the results of past studies [6,8,12,17]. After adjusting for age, sex, area of residence, smoking status, and drinking status, however, the association between HDL-C and all-cause mortality lost its statistical significance. To better determine the relationship between blood lipids and all-cause mortality, the model was further adjusted for BMI, SBP, DBP, blood urea nitrogen, plasma creatine, uric acid, and plasma glucose levels, and higher LDL-C and TG concentrations were found to be associated with lower all-cause mortality, which suggests that high LDL-C and TG levels may be independent protective factors related to all-cause mortality. The lowest mortality risk appeared in the third LDL-C (1.91-2.47 mmol/L) and fourth TG (≥ 1.66 mmol/L) quartiles. Several studies have found low LDL-C and TG levels to be associated with an increased mortality risk [6,12,18,19]. The possible mechanism for these relationships may be the protective role of lipids and lipoproteins in modulating inflammation markers, such as cytokines, C-reactive protein, and oxidized LDL-C [17,20,21]. Low-serum LDL-C increases the risk of infection and sepsis, leading to higher levels of oxidized LDL-C and increased inflammation. Furthermore, LDL-C functions as a carrier of the exogenous coenzyme Q10 which reduces the negative effects of septic shock by acting as an effective radical scavenger [22]. Relationships between low-serum LDL-C levels and the risk of fever, sepsis, and malignancy have been observed in studies of various populations [23-25]. However, the inverse relationship between LDL-C and TG levels and mortality may have been distorted by survival bias, meaning that the individuals most susceptible to the detrimental effects of high-serum cholesterol likely die before reaching a certain age and those who live longer (two-thirds of the study population aged 80 years and older) likely have a beneficial genetic risk profile related to LDL-C concerning familial longevity [2,26]. In addition, blood lipid concentration was found to be sensitive to dietary patterns, especially TG levels, which increase higher intake of dietary carbohydrates [27,28]. Therefore, older adults’ diets may be another potential contributing factor. The positive correlation between TC and all-cause mortality suggests this possibility. There are 2 possible explanations for this positive relationship. First, TC was identified as a risk factor for CHD, which remains a leading cause of death among older adults. Second, this study found that high levels of HDL-C increase the risk of mortality, although not significantly. Past studies have recommended an optimal range of HDL-C concentration related to all-cause mortality and have suggested that excessively high HDL-C levels may negatively affect the survival of older adults [8,29]. This negative effect may be stronger than the protective effect of normal HDL-C levels [29]. The sensitivity analysis revealed that model 4 had excellent discrimination ability, confirming this association.
- Blood lipid levels decline from middle-age to old-age [30,31]. Unlike Schupf et al. [6], who attributed the age difference in the relationship between LDL-C and mortality among older adults without dementia to chance, we assumed that the discrepancies in the results of past studies may be partly due to differences in the age limits of the older adults included in study populations [3,11,32,33]. In the current study, we further explored the relationship between blood lipids and all-cause mortality in different age groups. No significant relationships between TC, LDL-C, and TG and all-cause mortality were observed in the 60-80 years age group. Cabrera et al. [11] reported similar findings among Brazilian older adults aged 60-85 years. These results suggest that those in this age range may benefit from monitoring and controlling their lipid levels according to National Cholesterol Education Program guidelines to prevent cardiovascular disease. For those aged 80-100 years old, LDL-C and TG levels played a protective role against mortality and enhanced survival, indicating the importance of maintaining proper LDL-C levels (1.47-1.91 mmol/L) and high TG levels to live longer lives. The survival status of older adults aged 100 years and older did not seem to be affected by blood lipid levels and may depend to a large extent on their genetic profile [34,35].
- This study has several important limitations. First, due to constraints in human and material resources, we could not obtain biochemical information of older adults from all 23 regions. The final sample size and representativeness of the study population were not good enough to generalize the results of the study to the national elderly population. Second, it is very difficult to follow-up with elderly participants for 10 years, and the rate of loss to follow-up was relatively high (up to 26.0%) in this study. Third, although the model adjusted for blood pressure, BMI, and other factors, adjustments for several indicators that may also affect the survival status of older people, such as albumin (an indicator of frailty) and diet, were not included in the last model. Fourth, a relationship between blood lipid levels and all-cause mortality does not necessarily mean that blood lipids directly affect survival and death. We did not consider basic diseases, chronic diseases, or exposure to statins among the study population. Blood lipids may affect the course of a disease and ultimately affect survival and death or vice versa [36]. In addition, the cohort in this study was surveyed in the CLHLS and had a low prevalence of underlying diseases.
- In conclusion, monitoring blood lipid levels is important for a long and healthy life. Older people should be careful to maintain an LDL-C level of 1.91-2.47 mmol/L, and a TG level of 1.66 mmol/L may benefit the aging process. Future research on older adults is needed to understand discrepancies in the results of different studies.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Material 1.
Supplementary Material 2.
Supplementary Material 3.
Supplementary Material 4.
Supplementary Material 5.
Supplementary Material 6.
Supplementary Material 7.
Supplementary Material 8.
-
DATA AVAILABILITY
The datasets analyzed in this study are available at https://opendata.pku.edu.cn/dataset.xhtml?persistentId=doi:10.18170/DVN/WBO7LK
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
-
FUNDING
Shanghai Municipal Health Commission Clinical research project (202140203). The research was funded by the Shanghai 3-year Action Plan for Public Health under a grant (GWV-10.2-XD13, GWV-10.1-XK15, GWV-10.1-XK18), a strategic collaborative innovation team (SSMU-ZLCX20180601), and the National Key Research and Development Project (2018YFC1705100, 2018-YFC1705103).
-
AUTHOR CONTRIBUTIONS
Conceptualization: Cai Y, Hu F. Data curation: Hu F, Wang Z. Formal analysis: Wang Z. Funding acquisition: Cai Y, Wang Y, Shen T. Methodology: Chen H, Liu Y, Xu C, Chang R. Project administration: Xiang M, Xu G. Visualization: Xia D, Ge X, Chen Y. Writing – original draft: Wang R, Yu X, Wang Z. Writing – review & editing: Wang R, Yu X, Wang Z, Hu F, Cai Y, Liu Y, Chen H, Liu S, Xu C, Chen Y, Ge X, Xia D, Chang R, Xu G, Xiang M, Wang Y, Shen T.
NOTES
ACKNOWLEDGEMENTS
Values are presented as hazard ratio (95% confidence interval).
HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index.
1 Model 1: adjusted for age, sex, area of residence, marital status, economic status, smoking status, and drinking status; Model 2: further adjusted for SBP, DBP, and BMI based on model 1; Model 3: further adjusted for blood urea nitrogen, plasma creatine, uric acid, and plasma glucose based on model 2; Model 4: further adjusted for total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels based on model 3.
- 1. Yi SW, Yi JJ, Ohrr H. Total cholesterol and all-cause mortality by sex and age: a prospective cohort study among 12.8 million adults. Sci Rep 2019;9:1596.ArticlePubMedPMCPDF
- 2. Schatz IJ, Masaki K, Yano K, Chen R, Rodriguez BL, Curb JD. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet 2001;358:351-355.ArticlePubMed
- 3. Weverling-Rijnsburger AW, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RG. Total cholesterol and risk of mortality in the oldest old. Lancet 1997;350:1119-1123.ArticlePubMed
- 4. Jacobs D, Blackburn H, Higgins M, Reed D, Iso H, McMillan G, et al. Report of the Conference on Low Blood Cholesterol: mortality associations. Circulation 1992;86:1046-1060.ArticlePubMed
- 5. Yi SW, Park SJ, Yi JJ, Ohrr H, Kim H. High-density lipoprotein cholesterol and all-cause mortality by sex and age: a prospective cohort study among 15.8 million adults. Int J Epidemiol 2021;50:902-913.ArticlePubMedPDF
- 6. Schupf N, Costa R, Luchsinger J, Tang MX, Lee JH, Mayeux R. Relationship between plasma lipids and all-cause mortality in nondemented elderly. J Am Geriatr Soc 2005;53:219-226.ArticlePubMed
- 7. Dyer AR, Stamler J, Paul O, Shekelle RB, Schoenberger JA, Berkson DM, et al. Serum cholesterol and risk of death from cancer and other causes in three Chicago epidemiological studies. J Chronic Dis 1981;34:249-260.ArticlePubMed
- 8. Zhong GC, Huang SQ, Peng Y, Wan L, Wu YQ, Hu TY, et al. HDL-C is associated with mortality from all causes, cardiovascular disease and cancer in a J-shaped dose-response fashion: a pooled analysis of 37 prospective cohort studies. Eur J Prev Cardiol 2020;27:1187-1203.ArticlePubMedPDF
- 9. Pikhart H, Hubáček JA, Peasey A, Kubínová R, Bobák M. Association between fasting plasma triglycerides, all-cause and cardiovascular mortality in Czech population. Results from the HAPIEE study. Physiol Res 2015;64(Suppl 3):S355-S361.ArticlePubMed
- 10. Lv YB, Yin ZX, Chei CL, Qian HZ, Kraus VB, Zhang J, et al. Low-density lipoprotein cholesterol was inversely associated with 3-year all-cause mortality among Chinese oldest old: data from the Chinese Longitudinal Healthy Longevity Survey. Atherosclerosis 2015;239:137-142.ArticlePubMedPMC
- 11. Cabrera MA, de Andrade SM, Dip RM. Lipids and all-cause mortality among older adults: a 12-year follow-up study. ScientificWorldJournal 2012;2012:930139.ArticlePubMedPMCPDF
- 12. Tikhonoff V, Casiglia E, Mazza A, Scarpa R, Thijs L, Pessina AC, et al. Low-density lipoprotein cholesterol and mortality in older people. J Am Geriatr Soc 2005;53:2159-2164.ArticlePubMed
- 13. Goldbourt U, Holtzman E, Neufeld HN. Total and high density lipoprotein cholesterol in the serum and risk of mortality: evidence of a threshold effect. Br Med J (Clin Res Ed) 1985;290:1239-1243.ArticlePubMedPMC
- 14. Xiao Q, Wu M, Zeng T. Social support networks in Chinese older adults: health outcomes and health related behaviors: a path analysis. Aging Ment Health 2019;23:1382-1390.ArticlePubMedPMC
- 15. Yu J, Feng Q, Yu J, Zeng Y, Feng L. Late-life cognitive trajectories and their associated lifestyle factors. J Alzheimers Dis 2020;73:1555-1563.ArticlePubMedPMC
- 16. Sha S, Xu Y, Chen L. Loneliness as a risk factor for frailty transition among older Chinese people. BMC Geriatr 2020;20:300.ArticlePubMedPMCPDF
- 17. Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 2004;291:451-459.ArticlePubMed
- 18. Lv YB, Mao C, Gao X, Yin ZX, Kraus VB, Yuan JQ, et al. Triglycerides paradox among the oldest old: “the lower the better?”. J Am Geriatr Soc 2019;67:741-748.ArticlePubMedPMCPDF
- 19. Anderson KM, Castelli WP, Levy D. Cholesterol and mortality. 30 years of follow-up from the Framingham study. JAMA 1987;257:2176-2180.ArticlePubMed
- 20. Anand IS, Latini R, Florea VG, Kuskowski MA, Rector T, Masson S, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation 2005;112:1428-1434.ArticlePubMed
- 21. Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, et al. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol 2003;42:1933-1940.ArticlePubMed
- 22. Lelli JL, Drongowski RA, Gastman B, Remick DG, Coran AG. Effects of coenzyme Q10 on the mediator cascade of sepsis. Circ Shock 1993;39:178-187.PubMed
- 23. Shor R, Wainstein J, Oz D, Boaz M, Matas Z, Fux A, et al. Low serum LDL cholesterol levels and the risk of fever, sepsis, and malignancy. Ann Clin Lab Sci 2007;37:343-348.PubMed
- 24. Charach G, Rabinovich A, Ori A, Weksler D, Sheps D, Charach L, et al. Low levels of low-density lipoprotein cholesterol: a negative predictor of survival in elderly patients with advanced heart failure. Cardiology 2014;127:45-50.ArticlePubMedPDF
- 25. Kritchevsky SB, Kritchevsky D. Serum cholesterol and cancer risk: an epidemiologic perspective. Annu Rev Nutr 1992;12:391-416.ArticlePubMed
- 26. Postmus I, Deelen J, Sedaghat S, Trompet S, de Craen AJ, Heijmans BT, et al. LDL cholesterol still a problem in old age? A Mendelian randomization study. Int J Epidemiol 2015;44:604-612.ArticlePubMed
- 27. Schwingshackl L, Hoffmann G. Comparison of effects of long-term low-fat vs high-fat diets on blood lipid levels in overweight or obese patients: a systematic review and meta-analysis. J Acad Nutr Diet 2013;113:1640-1661.ArticlePubMed
- 28. Schwarz JM, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr 2003;77:43-50.ArticlePubMed
- 29. Li ZH, Lv YB, Zhong WF, Gao X, Byers Kraus V, Zou MC, et al. High-density lipoprotein cholesterol and all-cause and cause-specific mortality among the elderly. J Clin Endocrinol Metab 2019;104:3370-3378.ArticlePubMed
- 30. Slade E, Irvin MR, Xie K, Arnett DK, Claas SA, Kind T, et al. Age and sex are associated with the plasma lipidome: findings from the GOLDN study. Lipids Health Dis 2021;20:30.ArticlePubMedPMCPDF
- 31. Yi SW, An SJ, Park HB, Yi JJ, Ohrr H. Association between low-density lipoprotein cholesterol and cardiovascular mortality in statin non-users: a prospective cohort study in 14.9 million Korean adults. Int J Epidemiol 2022;51:1178-1189.ArticlePubMedPMCPDF
- 32. Reed D, Yano K, Kagan A. Lipids and lipoproteins as predictors of coronary heart disease, stroke, and cancer in the Honolulu Heart Program. Am J Med 1986;80:871-878.ArticlePubMed
- 33. Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA 1994;272:1335-1340.ArticlePubMed
- 34. Gentilini D, Mari D, Castaldi D, Remondini D, Ogliari G, Ostan R, et al. Role of epigenetics in human aging and longevity: genomewide DNA methylation profile in centenarians and centenarians’ offspring. Age (Dordr) 2013;35:1961-1973.ArticlePubMedPMCPDF
- 35. Brooks-Wilson AR. Genetics of healthy aging and longevity. Hum Genet 2013;132:1323-1338.ArticlePubMedPMCPDF
- 36. Iribarren C, Reed DM, Chen R, Yano K, Dwyer JH. Low serum cholesterol and mortality. Which is the cause and which is the effect? Circulation 1995;92:2396-2403.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Effects of Lipoproteins on Metabolic Health
Obaida Albitar, Crystal M. D’Souza, Ernest A. Adeghate
Nutrients.2024; 16(13): 2156. CrossRef

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite