Articles
- Page Path
- HOME > Epidemiol Health > Volume 44; 2022 > Article
-
Original Article
The association between fruit and vegetable consumption and metabolic syndrome in Korean adults: does multivitamin use matter? -
Jihae Kim1
 , Li-Juan Tan1
, Li-Juan Tan1 , Hyein Jung1
, Hyein Jung1 , Yumi Roh1
, Yumi Roh1 , Kyungjoon Lim2
, Kyungjoon Lim2 , Sangah Shin1
, Sangah Shin1
-
Epidemiol Health 2022;44:e2022039.
DOI: https://doi.org/10.4178/epih.e2022039
Published online: April 19, 2022
1Department of Food and Nutrition, Chung-Ang University, Anseong, Korea
2Faculty of medicine and Health, School of Medical Science, University of Sydney, Sydney, Australia
- Correspondence: Sangah Shin Department of Food and Nutrition, Chung-Ang University, 4726 Seodong-daero, Anseong 17546, Korea E-mail: ivory8320@cau.ac.kr
©2022, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
OBJECTIVES
- Metabolic syndrome (MetS) is closely associated with dietary intake; however, few studies have investigated whether the consumption of fruits and vegetables and multivitamin use affect MetS in the Korean population. This study aimed to examine these effects in Korean adults.
-
METHODS
- This was a cross-sectional study of 89,548 participants aged between 40 years and 69 years selected from the baseline data of the Health Examinees study conducted in Korea. Fresh vegetable and fruit consumption was assessed using a validated 106-item food frequency questionnaire. MetS and its components were defined using the National Cholesterol Education Program, Adult Treatment Panel III criteria. Multivariate logistic regression analyses were conducted to identify associations of fresh vegetable, fruit, and fresh vegetable+fruit consumption and multivitamin use with the prevalence of MetS.
-
RESULTS
- Female in the highest quartile of fresh vegetable, fruit, and fresh vegetable + fruit consumption exhibited a lower prevalence of MetS than those in the lowest quartile. An inverse association with the prevalence of MetS was observed among male with only fresh vegetable consumption. The interaction between the 3 categories and multivitamin intake on the prevalence of MetS was not significant (all pinteraction>0.05), regardless of sex.
-
CONCLUSIONS
- Multivitamin use and consumption of fresh vegetables and fruits had no significant synergistic effects. Although fresh vegetable and fruit consumption showed an inverse association with the prevalence of MetS, this relationship was not altered by multivitamin use.
- Keywords: Dietary behavior, Metabolic syndrome, Vitamins, Adults, Cohort studies
- Metabolic syndrome (MetS) is associated with the development of non-communicable diseases such as cardiovascular disease, type 2 diabetes mellitus, and stroke [1,2]. MetS affects 20-30% of the adult population in Europe [3], and its prevalence in adults in the United States approximately 36.4% [4]. Similarly, studies have shown an increasing prevalence of MetS in the Korean population (from 24.9% in 1998 to 28.9% in 2013) [5]. These data suggest that MetS is increasingly becoming a public health issue that also leads to increased medical costs and other socioeconomic burdens [6].
- Numerous studies have identified the risk factors for MetS in various domains, including investigations on lifestyle factors, physical activities, and dietary intake [7,8]. Studies have reported that the consumption of fruits and vegetables could reduce the risk of MetS [9,10]; a vegetarian diet was reported to be associated with a lower incidence of MetS in a previous study [11]. A meta-analysis also revealed that fruit and vegetable consumption was inversely associated with the risk of developing MetS [12].
- In Korea, daily fruit and vegetable consumption decreased from 35.7% in 2007 to 26.2% in 2018 according to the 2018 National Health Statistics [13]; reduced intake of fruits and vegetables is, in turn, associated with a reduction in the intake of vitamins and minerals [14]. Interestingly, there is instead an increasing trend in the consumption of dietary supplements such as multivitamins [15].
- According to studies based on animal models, clinical trials, and the biochemical analysis of new multivitamins, the intake of multivitamins with suitable proportions of vitamins B3 (niacin), B1 (thiamine), B2 (riboflavin), B6 (pyridoxine), and others with antioxidant activity can reduce symptom severity or the risk of chronic diseases, such as MetS [16]. It has also been reported that dietary supplementation with vitamin A (retinol and retinoic acid), vitamin E, and total antioxidant capacity vitamins decreased the prevalence of MetS and its component factors [17]. In this regard, there are insufficient findings in the literature to confirm whether the consumption of fruits and vegetables with concomitant use of multivitamins reduces the risk of MetS.
- Hence, in this study, we aimed to examine whether concomitant multivitamin use and the adequate consumption of fresh vegetables and fruits are associated with MetS and its five components among Korean adults aged 40 years to 69 years through a study design based on the Health Examinees (HEXA) study.
INTRODUCTION
- Study population
- This investigation was based on the HEXA study, a large-scale community-based genomic cohort study in Korea that was implemented from 2004 to 2013. The specific details of the HEXA study design have been thoroughly described elsewhere [18]. In total, 173,357 individuals aged 40 years to 69 years were initially included in this survey. Among this population, we excluded individuals who had missing values for the measures of certain components of MetS (n= 6,022), individuals who reported doubtful energy intake values (< 800 or ≥ 4,000 kcal/day in male, < 500 or ≥ 3,500 kcal/day in female; n= 3,132) [19], those who did not report the use of a multivitamin supplement (n= 69,455), and individuals who had missing values needed for the adjusting of possible confounding variables such as body mass index (BMI), household income, education level, smoking status, drinking status, and physical activity (n= 5,200). Ultimately, 89,548 participants (28,828 male and 60,720 female) were included in this study.
- Definition of metabolic syndrome
- MetS was defined in accordance with the National Cholesterol Education Program, Adult Treatment Panel III [20], and waist circumference (WC) criteria were based on the guidelines of the Korean Obesity Society [21]. Participants were diagnosed with MetS if they had 3 or more of the following 5 components: (1) increased WC, ≥ 90 cm in male and ≥ 85 cm in female; (2) elevated triglyceride (TG) levels, ≥ 150 mg/dL; (3) reduced levels of high-density lipoprotein cholesterol (HDL cholesterol), < 40 mg/dL in male and < 50 mg/dL in female; (4) elevated fasting blood glucose (FBG) levels, ≥ 100 mg/dL or using diabetes medication; (5) elevated blood pressure, systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg or using hypertension medication.
- Dietary assessment and multivitamin use
- Regular fruit and vegetable consumption was assessed using a self-administered 106-item food frequency questionnaire [22]. Participants reported the frequency and average amount of food or beverage items consumed during the previous year based on 3 standard serving sizes (0.5, 1.0, and 1.5 serving sizes). The consumption frequencies were classified into 14 categories, ranging from “never or seldom” to “3 times/day.”
- In the current study, we included 12 fruit items as fresh fruits: strawberries, muskmelons/melons, watermelons, peaches/plums, bananas, persimmons, tangerines, pears, apples, oranges, grapes, and tomatoes. We classified “vegetable wrap/vegetable salad” as involving fresh vegetables intake. Daily vegetable and fruit intake was calculated by multiplying the frequency of consumption by the specific serving sizes. Each vegetable and fruit item was used to classify consumption into 3 main groups: fresh vegetables+ fruits, fresh vegetables, and fruits.
- The total daily intake of energy and macronutrients (carbohydrates, proteins, and fats) were calculated using the food composition table of the Korean Health and Industry of Development Institute [23,24]. The amount or frequency of multivitamin use among the participants of this survey from 2004 to 2008 was classified into the following 6 categories: none, 1-3 pills or times/wk, 4-6 pills or times/wk, 1 pill or times/day, 2 pills or times/day, and ≥ 3 pills or times/day. For those who participated in this survey from 2009 to 2013, the amount or frequency of multivitamin use was calculated by multiplying the frequency of consumption by the amount per consumption. According to the results of the calculations (0 or not), participants were dichotomized as multivitamin users or non-users.
- Statistical analysis
- All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and a p-value < 0.05 was considered to indicate statistically significant. We performed analyses to estimate the odds ratios (ORs) and 95% confidence interval (CIs) of MetS and its components based on the quartiles (Q4) of fresh vegetable and fruit consumption by sex. Multivariable logistic regression analysis was performed after adjusting for potential confounders, such as age (continuous), BMI (except WC), household income (low, medium–low, medium–high, or high), education level (≤ elementary school, middle school, high school, or ≥ college), smoking status (never, past, or current), drinking status (never, past, or current), physical activity (yes or no), and energy intake (kcal/day, analyzed as a continuous variable). Linear trends across the quartiles were tested by assigning each participant the median value of the category and modeling the corresponding values as a continuous variable. Differences between categories were calculated using the chi-square test for categorical variables and generalized linear models for continuous variables. Stratified analyses were also performed to test whether observed associations relied on the use of multivitamins. In the stratified analyses, multivariable logistics models (adjusted for continuous and categorical confounders) were applied to assess the association between each 1-standard deviation (SD) increment of fruit and vegetable intake and MetS for multivitamin use. The p-value for interaction was tested by performing the likelihood ratio test. All these analyses were conducted separately by sex.
- Ethics statement
- All participants voluntarily signed an informed written consent form prior to enrollment. The current study was performed in accordance with the guidelines specified in the Declaration of Helsinki, and the study protocol was approved by the local Institutional Review Board of the Ethics Committee of the Korean Genome and Epidemiology Study of the Korea National Institute of Health (IRB No. E-1503-103-657).
MATERIALS AND METHODS
- The general characteristics of the study population by sex are presented in Table 1 in accordance with the quartiles of fresh vegetable and fruit consumption. Participants with higher fresh vegetable and fruit consumption also exhibited higher multivitamin intake, higher household income levels and education levels, and physical activity, and were less likely to be smokers or drinkers than those with the lowest fresh vegetable and fruit consumption (all p<0.001). Compared with male in the lowest quartile, those who were in the highest quartile of fresh vegetable and fruit intake were slightly older (p<0.001), whereas female in the highest quartile were slightly younger and had a higher BMI (p<0.001 and p=0.028, respectively). Analysis of daily energy and energy from macronutrients across the quartiles of the fresh vegetable and fruit consumption revealed that both male and female with higher fresh vegetable and fruit consumption had higher intakes of energy and energy proportions from proteins and fats than those with lower fresh vegetable and fruit consumption; however, energy intake from carbohydrates was inversely associated (all p<0.001). All selected covariates exhibited statistically significant differences among the fresh vegetable and fruit consumption categories (Table 1).
- The biomarkers and prevalence of MetS and its components according to the quartiles of fresh vegetable and fruit intake are presented in Table 2. Male with higher fresh vegetable and fruit consumption had the lower HDL cholesterol levels (p<0.001), as well as the lower SBP and DBP (p=0.004 and < 0.001, respectively); however, WC (p=0.567), TG (p=0.147) and FBG levels (p=0.713) were not significantly associated with the consumption of fresh vegetables and fruits. Except for HDL cholesterol levels, SBP and DBP, female with higher consumption of fresh vegetables and fruits had the lower mean WC, TG levels, and FBG levels (p values are 0.001, 0.017, and < 0.001, respectively).
- In the analysis of the prevalence of the 5 indicators of MetS in female, those who consumed more fresh vegetables and fruit had a lower prevalence of MetS and its five components (all p<0.001). However, in male, those who consumed more fresh vegetables and fruits only showed a lower prevalence of elevated TG levels (p=0.005), but a higher prevalence of reduced HDL cholesterol levels (p=0.020). Moreover, the prevalence of MetS (p=0.349), increased WC (p=0.970), or elevated FBG levels (p=0.717) did not show significant associations with fresh vegetable and fruit consumption (Table 2).
- The associations between fresh vegetable, fruit, or fresh vegetable+fruit consumption and the prevalence of MetS and its components are presented in Table 3. Female in the highest quartile of fresh vegetable, fruit, and fresh vegetable+fruit consumption had lower ORs of MetS (OR, 0.79; 95% confidence interval [CI], 0.74 to 0.85; p<0.001 in the fresh vegetable group, OR, 0.93; 95% CI, 0.87 to 0.99; p<0.001 in the fruit group, and OR, 0.91; 95% CI, 0.85 to 0.98; p<0.001 in the fresh vegetable and fruit group) than those in the lowest quartile. Higher fresh vegetable consumption was also associated with a lower OR of individual MetS components, including increased WC (OR, 0.83; 95% CI, 0.77 to 0.89; p<0.001), elevated TG levels (OR, 0.86; 95% CI, 0.80 to 0.92; p<0.001), and reduced HDL cholesterol levels (OR, 0.83; 95% CI, 0.78 to 0.87; p<0.001). Similarly, higher fruit and fresh vegetable+fruit consumption was associated with lower ORs of increased WC (OR, 0.88; 95% CI, 0.83 to 0.95; p<0.001 in the fruit group and OR, 0.88; 95% CI, 0.82 to 0.94; p<0.001 in the fresh vegetable+fruit group). Among male, a significant association was observed between higher fresh vegetable consumption and the prevalence of MetS (OR, 0.88; 95% CI, 0.81 to 0.96; p<0.001). However, there was no significant association between other consumption categories and the prevalence of MetS, and regarding the components of MetS, only elevated blood pressure exhibited lower prevalence than the group with the lowest consumption (OR, 0.90; 95% CI, 0.84 to 0.97; p<0.001 in the fruit group and OR, 0.90; 95% CI, 0.84 to 0.97; p<0.001 in the fresh vegetable+fruit group) (Table 3).
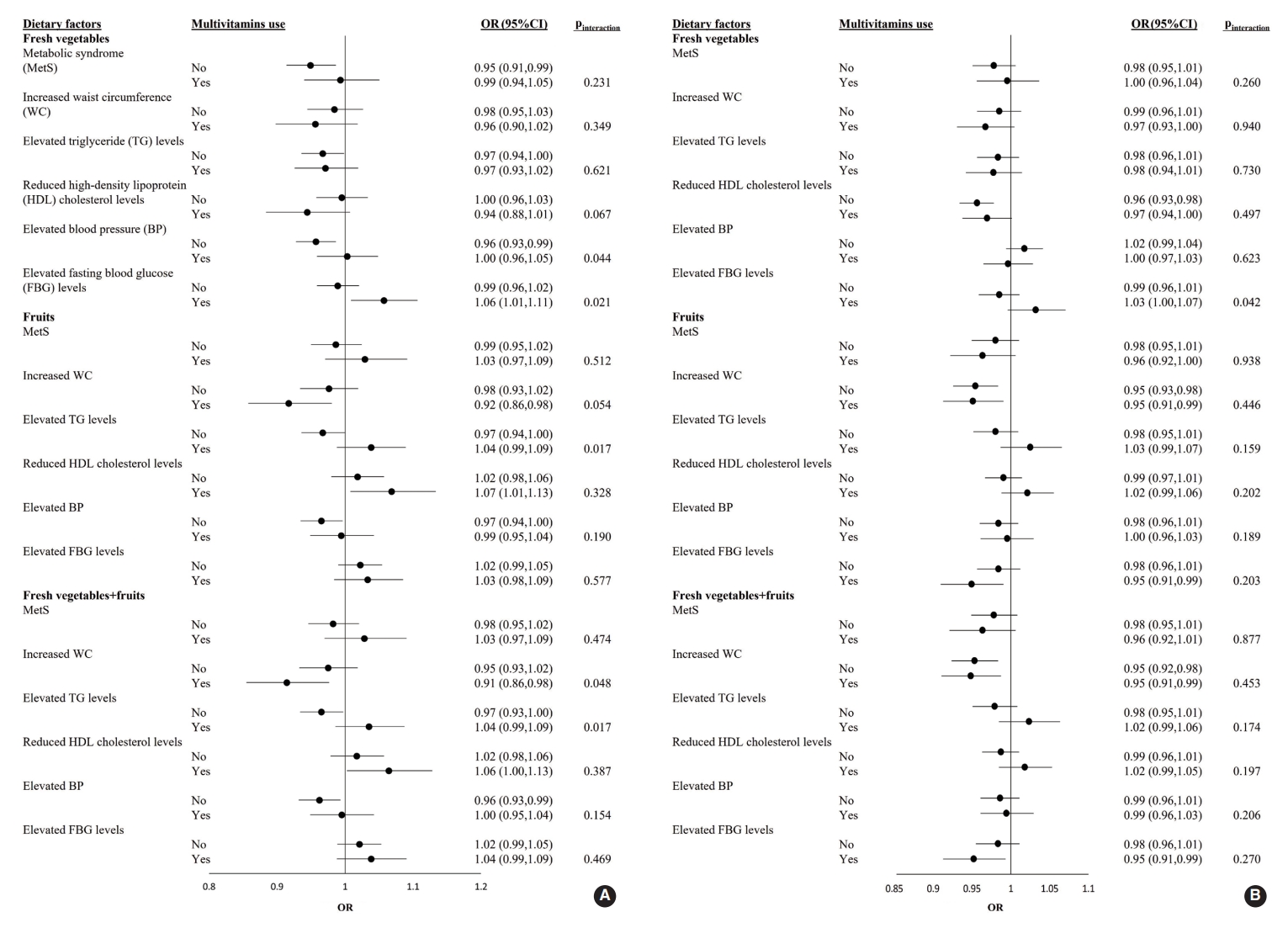
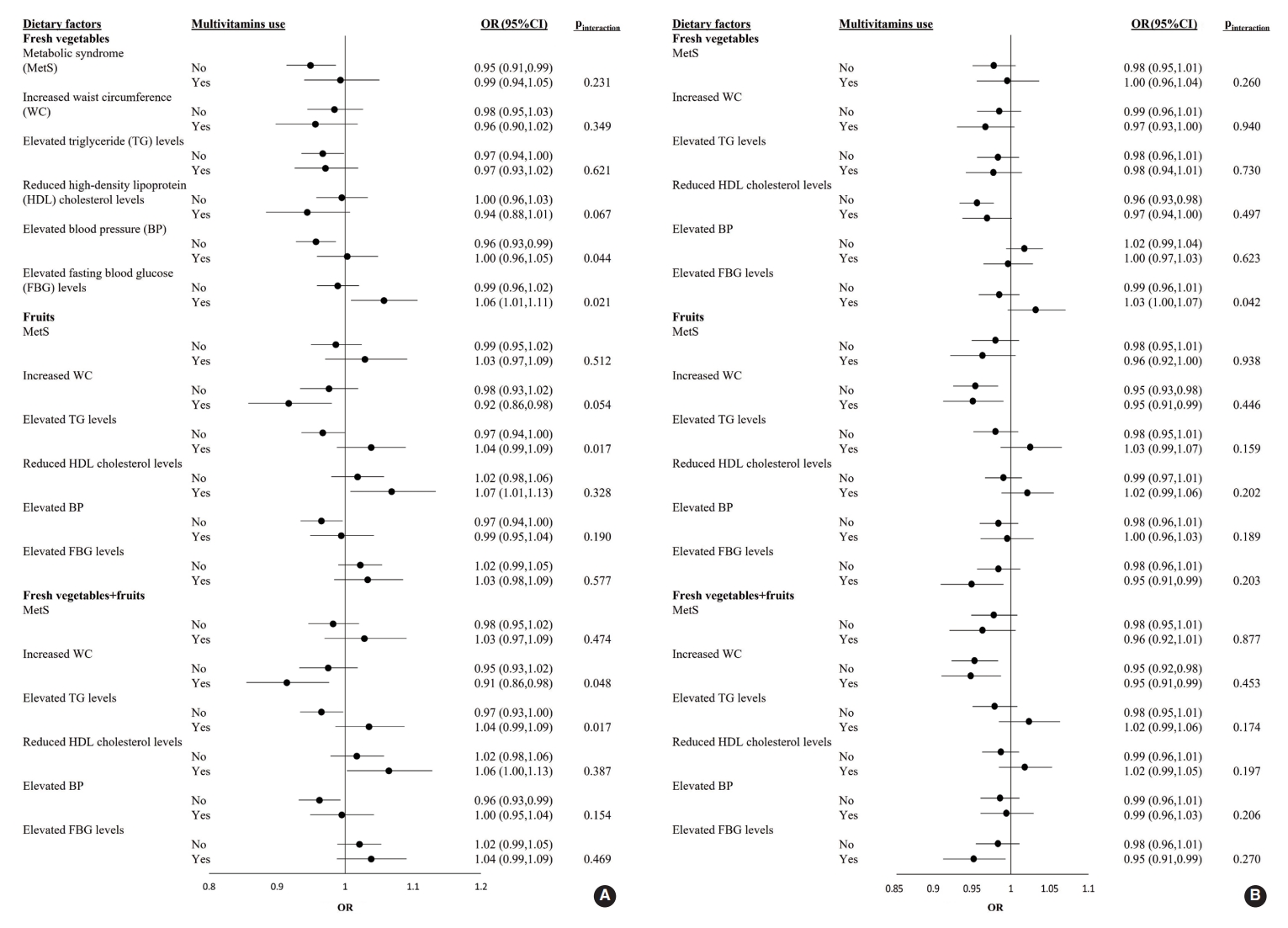
- The ORs and 95% CI of MetS for each 1-SD increment of fresh vegetable, fresh fruit, or fresh vegetable+fresh fruit consumption in male and female who were or were not taking multivitamins are shown in Figure 1. Among male participants, the adjusted OR for elevated FBG levels with a 1-SD increment in fresh vegetable intake was significantly higher in multivitamin users (OR, 1.06; 95% CI, 1.01 to 1.11) than in non-users (pinteraction=0.021). Regarding the effects of fresh vegetables+fruits on increased WC, the adjusted OR was significantly lower in multivitamin users as consumption increased (OR, 0.91; 95% CI, 0.85 to 0.98; pinteraction=0.048). Among female participants, there was no significant interaction for the prevalence of MetS and its 5 components between the 3 categories and multivitamin intake (all pinteraction>0.05) (Figure 1).
RESULTS
- In the current cross-sectional study, we discovered that fruit and fresh vegetable consumption had an association with a lower prevalence of MetS. Considering that kimchi (a traditional fermented vegetable in Korea) is very frequently consumed in Korea, we additionally adjusted the model for kimchi intake, and the cross-sectional association remained (Supplementary Material 1). However, concomitant multivitamin use did not alter the relationship in either male or female with adequate fruit and vegetable consumption.
- Our findings are similar to those of previous cross-sectional studies conducted in Korea that identified an inverse association between fruit consumption and the prevalence of MetS because of fruits’ phytochemicals and antioxidative vitamins [10,25]. Furthermore, several prospective epidemiological studies also demonstrated that a lower incidence of MetS was associated with higher fruit consumption due to the beneficial health-related components of fruits, such as antioxidants, phytochemicals, fiber, and minerals [26,27]. The slight difference in the association between vegetables and MetS compared with that in a previous Korean study may have resulted from the differences in vegetable inclusion criteria. In the current study, we included only fresh leafy vegetables and did not consider kimchi or tuber vegetables [10,26]. In a systematic review and meta-analysis, Shin et al. [28] reported that there were no significant associations between MetS and its 5 components and fruit and vegetable intake except for DBP. The result was inconsistent with ours, which may have been due to differences in subjects and exposures [28-32].
- Fruits and vegetables are essential, and they are rich in fiber, minerals, vitamins, and certain phytochemicals [33]. The risk of MetS may be amplified by antioxidant deficiency accompanied by oxidative stress, which may lead to oxidative changes in the extracellular space, promoting endothelial dysfunction and cardiovascular damage [34]. A higher intake of antioxidants could reduce the oxidative stress associated with MetS [35,36]. In addition, as phytochemicals can increase the body’s production of insulin [37], the components of fruits and vegetables may play a role in preventing insulin resistance associated with MetS [35].
- Although there are some multivitamin intake guidelines for certain populations, such as pregnant female, more recommendations or studies are needed for the general population [38]. The health benefits of multivitamin intake have been controversial. According to the National Institutes of Health State-of-the-Science Conference Statement, there is insufficient evidence to recommend multivitamins for the treatment or prevention of chronic diseases [39]. The Academy of Nutrition and Dietetics also noted in a 2009 position statement that there was no evidence that multivitamins are effective in preventing chronic disease and that it was preferable to maintain one’s health and to prevent chronic diseases by eating a variety of foods [40,41]. These statements are consistent with our findings that revealed no additional effects of multivitamin use in reducing the prevalence of MetS.
- In the current sex-stratified, cross-sectional analysis, overall, sex differences did not play a major role in the effects of the three categories on the prevalence of MetS, except that certain variables were more likely to show statistical significance among female than among male. According to a previous study, the slight difference may result from the differences in musculoskeletal and adipose tissue and sex hormone levels [42-44].
- The current study had some limitations. First, despite the finding of an inverse association between fresh vegetable and fruit intake and MetS, the limited cross-sectional data could not reveal causal associations. Second, we obtained the information regarding the quantity of fruits and vegetables consumed and multivitamin intake using a self-reported food frequency questionnaire, and there was no detailed information about whether the fruits and vegetables were processed; thus, dietary measurement errors were inevitable [45]. Finally, our study only included Korean adults aged 40-69 years; therefore, a complete analysis of Korean diets and their impact on health outcomes, such as MetS, would require a further evaluation with the inclusion of all age groups.
- Higher intake of fresh vegetables and fruits was associated with a lower overall prevalence of MetS. However, there was no significant synergistic effect of concomitant multivitamin use in those with adequate consumption of fresh vegetables and fruits. In the future, we will focus on the longitudinal association between fresh vegetable and fruit intake and the incidence of MetS to investigate the impact of improved eating habits, with an emphasis on the increased consumption of a variety of fresh vegetables and fruits, on the prevention of MetS rather than promoting the use of multivitamins.
DISCUSSION
SUPPLEMENTARY MATERIALS
Supplementary Material 1.
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
-
FUNDING
This research was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (NRF-2020R1C1C1014286).
-
AUTHOR CONTRIBUTIONS
Conceptualization: Shin S. Data curation: Shin S, Lim K. Formal analysis: Kim J, Tan LJ. Funding acquisition: Shin S. Methodology: Shin S, Tan LJ, Jung H. Project administration: Shin S. Visualization: Kim J, Tan LJ, Jung H, Roh Y. Writing – original draft: Kim J, Tan LJ, Jung H, Roh Y. Writing – review & editing: Tan LJ, Jung H, Shin S, Lim K, Kim J.
NOTES
ACKNOWLEDGEMENTS
| Variables1 | Q1 | Q2 | Q3 | Q4 | p-value | ||
|---|---|---|---|---|---|---|---|
| Male (n=28,828) | 7,207 | 7,207 | 7,207 | 7,207 | |||
| WC (cm) | 86.03±7.60 | 86.17±7.45 | 86.06±7.48 | 86.18±7.38 | 0.567 | ||
| TG level (mg/dL) | 155.20±120.50 | 152.61±113.93 | 149.43±104.65 | 145.69±100.89 | 0.143 | ||
| HDL cholesterol level (mg/dL) | 50.63±13.59 | 49.91±11.91 | 49.57±11.90 | 49.56±11.59 | <0.001 | ||
| FBG level (mg/dL) | 99.35±26.53 | 99.41±23.89 | 98.87±23.50 | 99.03±23.37 | 0.713 | ||
| SBP (mmHg) | 126.88±15.50 | 126.30±15.34 | 125.89±14.90 | 125.55±14.66 | 0.004 | ||
| DBP (mmHg) | 79.62±10.17 | 78.95±10.08 | 78.66±9.83 | 78.83±9.81 | <0.001 | ||
| Prevalence | |||||||
| MetS | 1,863 (25.8) | 1,813 (25.2) | 1,788 (24.8) | 1,776 (24.6) | 0.349 | ||
| Increased WC | 2,251 (31.2) | 2,265 (31.4) | 2,260 (31.4) | 2,278 (31.6) | 0.970 | ||
| Elevated TG levels | 2,753 (38.2) | 2,687 (37.3) | 2,664 (37.0) | 2,550 (35.4) | 0.005 | ||
| Reduced HDL cholesterol levels | 1,237 (17.2) | 1,315 (18.2) | 1,377 (19.1) | 1,341 (18.6) | 0.020 | ||
| Elevated FBG levels | 2,473 (34.3) | 2,490 (34.5) | 2,427 (33.7) | 2,473 (34.3) | 0.717 | ||
| Elevated BP | 3,427 (47.5) | 3,316 (46.0) | 3,242 (45.0) | 3,182 (44.1) | <0.001 | ||
| Female (n=60,720) | 15,180 | 15,180 | 15,180 | 15,180 | |||
| WC (cm) | 79.70±8.47 | 79.06±8.15 | 78.83±8.10 | 78.15±7.92 | 0.001 | ||
| TG level (mg/dL) | 118.66±79.80 | 114.12±74.04 | 112.09±72.83 | 111.36±73.06 | 0.017 | ||
| HDL cholesterol level (mg/dL) | 55.92±12.90 | 56.14±12.69 | 56.47±12.72 | 56.80±12.83 | 0.471 | ||
| FBG level (mg/dL) | 94.27±22.09 | 93.12±21.55 | 92.76±18.27 | 92.17±18.10 | <0.001 | ||
| SBP (mmHg) | 121.99±16.70 | 121.31±16.12 | 120.88±15.80 | 119.90±15.44 | 0.160 | ||
| DBP (mmHg) | 75.59±10.24 | 75.13±10.07 | 75.05±9.98 | 74.77±9.78 | 0.616 | ||
| Prevalence | |||||||
| MetS | 3,801 (25.0) | 3,449 (22.7) | 3,258 (21.5) | 2,890 (19.0) | <0.001 | ||
| Increased WC | 7,350 (48.4) | 6,894 (45.4) | 6,663 (43.9) | 6,171 (40.6) | <0.001 | ||
| Elevated TG levels | 3,481 (22.9) | 3,194 (21.0) | 3,038 (20.0) | 2,966 (19.5) | <0.001 | ||
| Reduced HDL cholesterol levels | 5,089 (33.5) | 4,943 (32.6) | 4,795 (31.6) | 4,640 (30.6) | <0.001 | ||
| Elevated FBG levels | 3,365 (22.2) | 3,090 (20.4) | 3,054 (20.1) | 2,752 (18.1) | <0.001 | ||
| Elevated BP | 5,155 (34.0) | 4,925 (32.4) | 4,756 (31.3) | 4,430 (29.2) | <0.001 | ||
Values are presented as adjusted mean±standard deviation or number (%).
MetS, metabolic syndrome; WC, waist circumference; TG, triglyceride; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; BP, blood pressure.
1 Increased WC: WC ≥90 cm in male, ≥85 cm in female; Elevated TG level: ≥150 mg/dL; Reduced HDL cholesterol level: <40 mg/dL in male, <50 mg/dL in female; Elevated BP: SBP ≥130 mmHg or DBP ≥85 mmHg or taking hypertensive medication; Elevated FBG level: fasting glucose ≥100 mg/dL or taking diabetes medication.
| Variables1,2 | Q1 | Q2 | Q3 | Q4 | p for trend | ||
|---|---|---|---|---|---|---|---|
| Male (n=28,828) | |||||||
| Fresh vegetables (n) | 7,012 | 7,039 | 6,661 | 8,116 | |||
| Range (Min-Max), g/day | 0.00–0.00 | 0.83–2.50 | 4.17–6.25 | 10.71–225.00 | |||
| MetS | 1.00 (reference) | 1.03 (0.95, 1.12) | 0.91 (0.83, 0.99) | 0.88 (0.81, 0.96) | <0.001 | ||
| Increased WC | 1.00 (reference) | 0.95 (0.86, 1.05) | 0.86 (0.78, 0.95) | 0.86 (0.78, 0.95) | 0.003 | ||
| Elevated TG levels | 1.00 (reference) | 0.97 (0.90, 1.04) | 1.00 (0.93, 1.08) | 0.97 (0.90, 1.05) | 0.002 | ||
| Reduced HDL cholesterol levels | 1.00 (reference) | 1.02 (0.93, 1.11) | 0.99 (0.91, 1.09) | 1.04 (0.95, 1.13) | 0.435 | ||
| Elevated BP | 1.00 (reference) | 0.95 (0.88, 1.01) | 0.94 (0.87, 1.01) | 0.87 (0.82, 0.94) | <0.001 | ||
| Elevated FBG levels | 1.00 (reference) | 1.04 (0.97, 1.12) | 1.02 (0.94, 1.09) | 1.01 (0.94, 1.08) | 0.742 | ||
| Fruits (n) | 7,207 | 7,207 | 7,207 | 7,207 | |||
| Range (Min-Max), g/day | 0.00–52.50 | 52.50–114.90 | 114.91–223.54 | 223.60–3,410.00 | |||
| MetS | 1.00 (reference) | 0.96 (0.89, 1.05) | 1.01 (0.92, 1.09) | 0.99 (0.90, 1.08) | <0.001 | ||
| Increased WC | 1.00 (reference) | 1.00 (0.91, 1.10) | 1.01 (0.91, 1.11) | 0.95 (0.85, 1.05) | 0.002 | ||
| Elevated TG levels | 1.00 (reference) | 0.97 (0.90, 1.04) | 1.00 (0.93, 1.08) | 0.97 (0.90, 1.05) | 0.002 | ||
| Reduced HDL cholesterol levels | 1.00 (reference) | 1.07 (0.98, 1.17) | 1.21 (1.11, 1.32) | 1.15 (1.05, 1.26) | 0.442 | ||
| Elevated BP | 1.00 (reference) | 0.95 (0.88, 1.01) | 0.94 (0.88, 1.01) | 0.90 (0.84, 0.97) | <0.001 | ||
| Elevated FBG levels | 1.00 (reference) | 1.00 (0.93, 1.07) | 0.99 (0.93, 1.07) | 1.018 (0.94, 1.10) | 0.767 | ||
| Fresh vegetables and fruits (n) | 7,207 | 7,207 | 7,207 | 7,207 | |||
| Range (Min-Max), g/day | 0.00–57.75 | 57.77–122.28 | 122.28–233.13 | 233.15–3,460.00 | |||
| MetS | 1.00 (reference) | 0.97 (0.90, 1.06) | 1.00 (0.92, 1.09) | 0.98 (0.90, 1.07) | <0.001 | ||
| Increased WC | 1.00 (reference) | 0.98 (0.89, 1.08) | 1.03 (0.93, 1.13) | 0.93 (0.84, 1.03) | 0.002 | ||
| Elevated TG levels | 1.00 (reference) | 0.99 (0.92, 1.06) | 1.01 (0.94, 1.09) | 0.97 (0.90, 1.05) | 0.002 | ||
| Reduced HDL cholesterol levels | 1.00 (reference) | 1.10 (1.01, 1.20) | 1.18 (1.08, 1.29) | 1.16 (1.05, 1.27) | 0.443 | ||
| Elevated BP | 1.00 (reference) | 0.95 (0.89, 1.02) | 0.93 (0.87, 1.00) | 0.90 (0.84, 0.97) | <0.001 | ||
| Elevated FBG levels | 1.00 (reference) | 1.02 (0.95, 1.09) | 0.99 (0.92, 1.07) | 1.02 (0.94, 1.10) | 0.768 | ||
| Female (n=60,720) | |||||||
| Fresh vegetables (n) | 15,317 | 18,857 | 15,159 | 11,387 | |||
| Range (Min-Max), g/day | 0.00–0.83 | 1.67–4.17 | 5.36–10.71 | 12.50–225.00 | |||
| MetS | 1.00 (reference) | 0.89 (0.84, 0.94) | 0.89 (0.84, 0.95) | 0.79 (0.74, 0.85) | <0.001 | ||
| Increased WC | 1.00 (reference) | 0.88 (0.83, 0.94) | 0.89 (0.84, 0.95) | 0.83 (0.77, 0.89) | <0.001 | ||
| Elevated TG levels | 1.00 (reference) | 0.93 (0.88, 0.98) | 0.93 (0.87, 0.98) | 0.86 (0.80, 0.92) | <0.001 | ||
| Reduced HDL cholesterol levels | 1.00 (reference) | 0.94 (0.90, 0.98) | 0.92 (0.87, 0.96) | 0.83 (0.78, 0.87) | <0.001 | ||
| Elevated BP | 1.00 (reference) | 1.02 (0.97, 1.07) | 1.00 (0.95, 1.05) | 1.01 (0.95, 1.07) | 0.927 | ||
| Elevated FBG levels | 1.00 (reference) | 0.93 (0.88, 0.98) | 0.93 (0.87, 0.98) | 0.94 (0.88, 1.00) | 0.241 | ||
| Fruits (n) | 15,180 | 15,180 | 15,180 | 15,180 | |||
| Range (Min-Max), g/day | 0.00–83.65 | 83.66–173.63 | 173.63–323.93 | 323.93–4,480.00 | |||
| MetS | 1.00 (reference) | 1.02 (0.96, 1.08) | 0.97 (0.91, 1.03) | 0.93 (0.87, 0.99) | <0.001 | ||
| Increased WC | 1.00 (reference) | 0.97 (0.91, 1.03) | 0.92 (0.86, 0.98) | 0.88 (0.83, 0.95) | <0.001 | ||
| Elevated TG levels | 1.00 (reference) | 0.97 (0.92, 1.03) | 0.94 (0.90, 1.00) | 0.98 (0.92, 1.04) | <0.001 | ||
| Reduced HDL cholesterol levels | 1.00 (reference) | 1.04 (0.99, 1.10) | 1.00 (0.95, 1.05) | 1.03 (0.97, 1.08) | <0.001 | ||
| Elevated BP | 1.00 (reference) | 1.03 (0.98, 1.09) | 1.00 (0.95, 1.06) | 0.97 (0.91, 1.02) | 0.994 | ||
| Elevated FBG levels | 1.00 (reference) | 0.97 (0.92, 1.03) | 0.98 (0.92, 1.04) | 0.92 (0.87, 0.98) | 0.144 | ||
| Fresh vegetables and fruits (n) | 15,180 | 15,180 | 15,180 | 15,180 | |||
| Range (Min-Max), g/day | 0.00–90.27 | 90.27–182.95 | 182.95–334.91 | 334.91–4,481.67 | |||
| MetS | 1.00 (reference) | 1.00 (0.94, 1.06) | 0.95 (0.90, 1.01) | 0.91 (0.85, 0.98) | <0.001 | ||
| Increased WC | 1.00 (reference) | 0.96 (0.90, 1.02) | 0.91 (0.85, 0.97) | 0.88 (0.82, 0.94) | <0.001 | ||
| Elevated TG levels | 1.00 (reference) | 0.97 (0.92, 1.03) | 0.94 (0.88, 0.99) | 0.98 (0.92, 1.04) | <0.001 | ||
| Reduced HDL cholesterol levels | 1.00 (reference) | 1.02 (0.97, 1.07) | 1.00 (0.95, 1.05) | 1.01 (0.95, 1.06) | <0.001 | ||
| Elevated BP | 1.00 (reference) | 1.03 (0.98, 1.09) | 1.00 (0.95, 1.06) | 0.97 (0.91, 1.02) | 0.996 | ||
| Elevated FBG levels | 1.00 (reference) | 0.97 (0.92, 1.03) | 0.99 (0.93, 1.05) | 0.93 (0.87, 0.99) | 0.143 | ||
Values are presented as adjusted odds ratio (95% confidence interval).
Model was adjusted for age, body mass index, educational level, physical activity, smoking status, drinking status and total energy intake.
MetS, metabolic syndrome; Min, minimum; Max, maximum; WC, waist circumference; TG, triglyceride; HDL, high-density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; BP, blood pressure.
1 Fresh vegetables: only fresh vegetable; Fruits: only fruit; Fresh vegetables and fruits: both fresh vegetables+fruits.
2 Increased WC: WC ≥90 cm in male, ≥85 cm in female; Elevated TG level: ≥150 mg/dL; Reduced HDL cholesterol level, <40 mg/dL in male, <50 mg/dL in female; Elevated BP: SBP ≥130 mmHg or DBP ≥85 mmHg or taking hypertensive medication; Elevated FBG level: fasting glucose ≥100 mg/dL or taking diabetes medication.
- 1. Ju SY, Lee JY, Kim DH. Association of metabolic syndrome and its components with all-cause and cardiovascular mortality in the elderly: a meta-analysis of prospective cohort studies. Medicine (Baltimore) 2017;96:e8491.PubMedPMC
- 2. Li X, Li X, Lin H, Fu X, Lin W, Li M, et al. Metabolic syndrome and stroke: a meta-analysis of prospective cohort studies. J Clin Neurosci 2017;40:34-38.ArticlePubMed
- 3. Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol 2008;28(4):629-636.ArticlePubMed
- 4. Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes 2010;2(3):180-193.ArticlePubMed
- 5. Shin S, Lee HW, Kim CE, Lim J, Lee JK, Kang D. Association between milk consumption and metabolic syndrome among Korean adults: results from the Health Examinees Study. Nutrients 2017;9:1102.ArticlePubMedPMC
- 6. Curtis LH, Hammill BG, Bethel MA, Anstrom KJ, Gottdiener JS, Schulman KA. Costs of the metabolic syndrome in elderly individuals: findings from the Cardiovascular Health Study. Diabetes Care 2007;30:2553-2558.PubMed
- 7. Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med 2004;34:371-418.PubMed
- 8. Hu T, Mills KT, Yao L, Demanelis K, Eloustaz M, Yancy WS Jr, et al. Effects of low-carbohydrate diets versus low-fat diets on metabolic risk factors: a meta-analysis of randomized controlled clinical trials. Am J Epidemiol 2012;176(Suppl 7):S44-S54.ArticlePubMedPMC
- 9. de Oliveira EP, McLellan KC, Vaz de Arruda Silveira L, Burini RC. Dietary factors associated with metabolic syndrome in Brazilian adults. Nutr J 2012;11:13.ArticlePubMedPMCPDF
- 10. Park S, Ham JO, Lee BK. Effects of total vitamin A, vitamin C, and fruit intake on risk for metabolic syndrome in Korean women and men. Nutrition 2015;31(1):111-118.ArticlePubMed
- 11. Sabaté J, Wien M. A perspective on vegetarian dietary patterns and risk of metabolic syndrome. Br J Nutr 2015;113 Suppl 2:S136-S143.PubMed
- 12. Tian Y, Su L, Wang J, Duan X, Jiang X. Fruit and vegetable consumption and risk of the metabolic syndrome: a meta-analysis. Public Health Nutr 2018;21:756-765.ArticlePubMed
- 13. Korea Centers for Disease Control and Prevention. The 2018 national health statistics: Korea National Health and Nutrition Examination Survey (KNHANES); 2020 [cited 2021 Jun 12]. Available from: https://knhanes.kdca.go.kr/knhanes/sub04/sub04_04_01.do (Korean).
- 14. Abdel-Rahman A, Anyangwe N, Carlacci L, Casper S, Danam RP, Enongene E, et al. The safety and regulation of natural products used as foods and food ingredients. Toxicol Sci 2011;123:333-348.ArticlePubMed
- 15. Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med 2013;173:355-361.ArticlePubMed
- 16. Wu SX, Jiang X, Liu YY, Chen LF, Tao J. Effects and mechanisms of a new multivitamin on chronic metabolic syndromes and aging. Afr J Tradit Complement Altern Med 2016;14:52-61.ArticlePubMedPMC
- 17. Kim S, Song Y, Lee JE, Jun S, Shin S, Wie GA, et al. Total antioxidant capacity from dietary supplement decreases the likelihood of having metabolic syndrome in Korean adults. Nutrients 2017;9:1055.ArticlePubMedPMC
- 18. Health Examinees Study Group. The Health Examinees (HEXA) study: rationale, study design and baseline characteristics. Asian Pac J Cancer Prev 2015;16:1591-1597.ArticlePubMed
- 19. Shin S, Lee HW, Kim CE, Lim J, Lee JK, Lee SA, et al. Egg consumption and risk of metabolic syndrome in Korean adults: results from the Health Examinees Study. Nutrients 2017;9:687.ArticlePubMedPMC
- 20. Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program - Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 2007;30:8-13.ArticlePubMedPDF
- 21. Lee S, Park HS, Kim SM, Kwon HS, Kim DY, Kim DJ, et al. Cutoff points of waist circumference for defining abdominal obesity in the Korean population. Korean J Obes 2006;15:1-9 (Korean).
- 22. Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr 2007;61:1435-1441.ArticlePubMedPDF
- 23. National Institute of Agricultural Sciences. Korean standard food composition table: 9th revision. [cited 2021 Jun 12]. Available from: http://koreanfood.rda.go.kr/eng/fctFoodSrchEng/engMain.
- 24. Park SH, Kim SN, Lee SH, Choe JS, Choi Y. Development of 9th revision Korean food composition table and its major changes. Korean J Community Nutr 2018;23:352-365 (Korean).ArticlePDF
- 25. Kim OY, Kwak SY, Kim B, Kim YS, Kim HY, Shin MJ. Selected food consumption mediates the association between education level and metabolic syndrome in Korean adults. Ann Nutr Metab 2017;70:122-131.ArticlePubMedPDF
- 26. Lim M, Kim J. Association between fruit and vegetable consumption and risk of metabolic syndrome determined using the Korean Genome and Epidemiology Study (KoGES). Eur J Nutr 2020;59:1667-1678.ArticlePubMedPDF
- 27. Baik I, Lee M, Jun NR, Lee JY, Shin C. A healthy dietary pattern consisting of a variety of food choices is inversely associated with the development of metabolic syndrome. Nutr Res Pract 2013;7:233-241.ArticlePubMedPMC
- 28. Shin JY, Kim JY, Kang HT, Han KH, Shim JY. Effect of fruits and vegetables on metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. Int J Food Sci Nutr 2015;66:416-425.ArticlePubMed
- 29. Mulero J, Bernabé J, Cerdá B, García-Viguera C, Moreno DA, Albaladejo MD, et al. Variations on cardiovascular risk factors in metabolic syndrome after consume of a citrus-based juice. Clin Nutr 2012;31:372-377.ArticlePubMed
- 30. Lehtonen HM, Suomela JP, Tahvonen R, Vaarno J, Venojärvi M, Viikari J, et al. Berry meals and risk factors associated with metabolic syndrome. Eur J Clin Nutr 2010;64:614-621.ArticlePubMedPDF
- 31. Basu A, Fu DX, Wilkinson M, Simmons B, Wu M, Betts NM, et al. Strawberries decrease atherosclerotic markers in subjects with metabolic syndrome. Nutr Res 2010;30:462-469.ArticlePubMedPMC
- 32. Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr 2010;140:1582-1587.ArticlePubMedPMC
- 33. Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr 2012;3:506-516.ArticlePubMedPMC
- 34. Couillard C, Ruel G, Archer WR, Pomerleau S, Bergeron J, Couture P, et al. Circulating levels of oxidative stress markers and endothelial adhesion molecules in men with abdominal obesity. J Clin Endocrinol Metab 2005;90:6454-6459.ArticlePubMed
- 35. Ando K, Fujita T. Metabolic syndrome and oxidative stress. Free Radic Biol Med 2009;47:213-218.ArticlePubMed
- 36. Lee M, Lim M, Kim J. Fruit and vegetable consumption and the metabolic syndrome: a systematic review and dose-response meta-analysis. Br J Nutr 2019;122:723-733.ArticlePubMed
- 37. Jayaprakasam B, Vareed SK, Olson LK, Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem 2005;53:28-31.ArticlePubMed
- 38. Cueto HT, Riis AH, Hatch EE, Wise LA, Rothman KJ, Mikkelsen EM. Predictors of preconceptional folic acid or multivitamin supplement use: a cross-sectional study of Danish pregnancy planners. Clin Epidemiol 2012;4:259-265.ArticlePubMedPMC
- 39. National Institutes of Health State-of-the-Science Panel. National Institutes of Health State-of-the-Science Conference Statement: multivitamin/mineral supplements and chronic disease prevention. Am J Clin Nutr 2007;85:257S-264S.ArticlePubMed
- 40. Marra MV, Boyar AP. Position of the American Dietetic Association: nutrient supplementation. J Am Diet Assoc 2009;109:2073-2085.ArticlePubMed
- 41. White ND. Messaging and multivitamin use: rethinking the “it can’t hurt” philosophy. Am J Lifestyle Med 2019;13:243-245.ArticlePubMedPMCPDF
- 42. Huang G, Cherkerzian S, Loucks EB, Buka SL, Handa RJ, Lasley BL, et al. Sex differences in the prenatal programming of adult metabolic syndrome by maternal androgens. J Clin Endocrinol Metab 2018;103:3945-3953.ArticlePubMed
- 43. Alvero-Cruz JR, Fernández Vázquez R, Martínez Blanco J, Diaz AJ, Rosety I, Rosety MA, et al. Sex differences for predicting metabolic syndrome by adipose dysfunction markers in institutionalized elderly. Eur J Cardiovasc Nurs 2021;20:534-539.ArticlePubMedPDF
- 44. Yoshimoto T, Ochiai H, Shirasawa T, Nagahama S, Uehara A, Sai S, et al. Sex differences in the association of metabolic syndrome with low back pain among middle-aged Japanese adults: a large-scale cross-sectional study. Biol Sex Differ 2019;10:33.ArticlePubMedPMCPDF
- 45. Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food-frequency questionnaires - a review. Public Health Nutr 2002;5:567-587.ArticlePubMed
REFERENCES
Figure & Data
References
Citations

- Association of serum water-soluble vitamin exposures with the risk of metabolic syndrome: results from NHANES 2003-2006
Xun Pei, Junjie Yao, Simiao Ran, Haifei Lu, Shuo Yang, Yini Zhang, Miyuan Wang, Heyuan Shi, Aihua Tan
Frontiers in Endocrinology.2023;[Epub] CrossRef

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite