Articles
- Page Path
- HOME > Epidemiol Health > Volume 38; 2016 > Article
-
Original Article
Age-period-cohort analysis of hepatitis A incidence rates in Korea from 2002 to 2012 -
Joo Yeon Seo1*
 , Sungyong Choi2*
, Sungyong Choi2* , BoYoul Choi1
, BoYoul Choi1 , Moran Ki3
, Moran Ki3
-
Epidemiol Health 2016;38:e2016040.
DOI: https://doi.org/10.4178/epih.e2016040
Published online: September 30, 2016
1Department of Preventive Medicine & Institute for Health and Society, Hanyang University College of Medicine, Seoul, Korea
2Gyeonggi Infectious Disease Control Center, Seongnam, Korea
3Department of Cancer Control and Policy, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea
- Correspondence: Moran Ki Department of Cancer Control and Policy, Graduate School of Cancer Science and Policy, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang 10408, Korea Tel: +82-31-920-2736, Fax: +82-50-4069-4908, Email: moranki@ncc.re.kr
- *Seo and Choi have contributed equally to this work as joint first authors.
• Received: September 1, 2016 • Revised: September 30, 2016 • Accepted: September 30, 2016
© 2016, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
OBJECTIVES
- This study aimed to evaluate the epidemiology of hepatitis A in Korea from 2002 to 2012 using age-period-cohort analyses.
-
METHODS
- We used claims data from the Korean National Health Insurance Corporation for the entire population. Census data from 2010 were used as the standard population. The incidence of hepatitis A was assumed to have a Poisson distribution, and the models and effects were evaluated using the intrinsic estimator method, the likelihood ratio, and the Akaike information criterion.
-
RESULTS
- The incidence of hepatitis A gradually increased until 2007 (from 17.55 to 35.72 per 100,000 population) and peaked in 2009 (177.47 per 100,000 population). The highest incidence was observed among 27-29-year-old individuals when we omitted data from 2005 to 2007. From 2005 to 2007, the peak incidence was observed among 24-26-year-old individuals, followed by 27-29-year-olds. The best model fits were observed when the age-period-cohort variables were all considered at the same time for males, females, and the whole population.
-
CONCLUSIONS
- The incidence of hepatitis A exhibited significant age-period-cohort effects; its incidence peaked in 2009 and was especially high among Koreans 20-39 years of age. These epidemiological patterns may help predict when high incidence rates of hepatitis A may occur in developing countries during their socioeconomic development.
- Hepatitis A virus (HAV) is mainly transmitted through the fecal-oral route, although transmission can also involve eating contaminated food or person-to-person transmission [1,2]. Lifelong immunity is often acquired after HAV infection [3], and hepatitis A during childhood is generally asymptomatic or causes flu-like symptoms. However, it is also associated with symptoms that range from nausea and vomiting to fulminant hepatitis and death among adults [1,2,4,5]. Unfortunately, hepatitis A is one of the most common infectious diseases in the world [6], and its incidence varies according to socioeconomic development and public sanitation. The endemicity of hepatitis A is generally high or intermediate in developing countries [6-8], and low in developed countries [8]. Furthermore, epidemiological shifts can occur within a country or birth cohort group, based on socioeconomic developments and public sanitation improvements [7]. Moreover, these characteristics are pronounced in countries that have experienced high levels of socioeconomic growth, such as South Korea (hereafter Korea) [7,8]. Therefore, it is important to evaluate the incidence patterns of hepatitis A according to age, period, and birth cohort, in order to understand such epidemiological shifts and to develop suitable public policy initiatives. This study aimed to determine the epidemiological characteristics of hepatitis A in Korea from 2002 to 2012, based on age-period-cohort (APC) analyses.
INTRODUCTION
- Ethical statement
- The retrospective design of this study was reviewed and approved by the institutional review board of Hanyang University (HYI-15-024-2).
- Data source
- We used claims data from the Korean National Health Insurance Corporation. These data included sex, age, the patient’s address, disease type, date of diagnosis, and medical history. Cases of hepatitis A were identified using the International Classification of Diseases, 10th revision codes B15, B15.0, and B15.9. In cases of repeated treatment for the same diagnosis, the first claim was used for the analyses. The annual mid-year populations were provided by Statistics Korea.
- Statistical analysis
- Population and housing census data from 2010 were used as the standard population for calculating the age-standardized incidence of hepatitis A. APC analyses were used to identify the age, period, and cohort effects of hepatitis A. The category of age was divided into 3-year groups, with the exception of a ≥81-year-old group based on the low incidence of hepatitis A in that group. The time periods were defined as 2002-2004, 2005-2007, 2008-2010, and 2011-2012. Birth cohorts were defined on the basis of 3-year cohorts from 1922 to 2012, and individuals who were born before 1921 were included in a single cohort. The incidence of hepatitis A was assumed to have a Poisson distribution, and the APC effects were measured using the intrinsic estimator (IE) method [9]. The optimal model was selected based on the likelihood ratio and the Akaike information criterion. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
MATERIALS AND METHODS
- Hepatitis A incidence gradually increased starting from 2002, and peaked at 83,414 individuals in 2009 (Table 1). The patterns of incidence according to sex were similar. The overall incidence according to age from 2002 to 2012 was highest in individuals 30-39 years old (102,065 individuals), followed by 20-29-year-olds (93,175 individuals).
- Table 2 presents the age-standardized hepatitis A incidence per 100,000 population according to sex and birth year from 2002 to 2012. In 2009, the incidence of hepatitis A in the overall population and males peaked in the 1978-1980 cohort (29-31 years old; 459.99 and 529.46 per 100,000 population, respectively). The incidence in the 1981-1983 cohort of females (26-28 years old) peaked at 408.43 per 100,000 population. The 1975-1986 cohorts (25-35 years old) generally exhibited the highest incidence rates, although some differences were found between males and females in the peak incidence rates according to cohort and age at diagnosis.
- Figure 1 shows the incidence of hepatitis A during from 2002 to 2012; it gradually increased from 2002 to 2007 (from 17.55 to 35.72 per 100,000 population, respectively) and peaked in 2009 (177.47 per 100,000 population). The incidence subsequently decreased to 67.16 per 100,000 population in 2012. When we omitted the data from 2005 to 2007, the highest incidence was observed among 27-29-year-old individuals. From 2005 to 2007, the highest incidence was observed for 24-26-year-old individuals, followed by individuals who were 27-29 years old. The incidence rates for males and females were similar, although they were slightly higher for men (Figure 2).
- The APC analyses revealed that the best model fits for males, females, and the overall populations were observed when all APC variables were considered at the same time (Table 3). Figure 3 shows the APC effect using IE models. After correcting for the period and cohort effects, a high incidence was observed for 0-2-year-old individuals, and the incidences subsequently decreased until the age of 6-8 years. The incidence rates gradually increased starting with the age of 9-11 years, peaked in the late 30s, and subsequently decreased with age (Figure 3A). After correcting for age and cohort effects, the incidence of hepatitis A was found to have increased from 2002-2004 through 2008-2010, and then decreased beginning in 2011. Similar patterns were observed for males and females (Figure 3B). After correcting for age and period effects, the incidence of hepatitis A was highest in the pre-1921 birth cohort, decreased until the 1957-1959 cohort, and subsequently increased until the 1999-2001 cohort (Figure 3C).
RESULTS
- We performed APC analyses of hepatitis A in Korea from 2002 to 2012 using nationally representative data. These analyses revealed that the full APC model provided a better fit than the age, period, age-period, age-cohort, and period-cohort models. Therefore, the APC model was best suited for our analysis.
- Korea is a good example of the transition from a poor country to a developing country and subsequently to a developed country; these transitions influenced public health issues, such as hepatitis A epidemiology. For example, the per capita Korean gross domestic product remained at <200 USD between the Korean War (1950-1953) and 1968, although it has subsequently increased to approximately 27,000 USD in 2015. This economic development and concomitant improvements in public sanitation have altered the epidemiology of hepatitis A in Korea, and the endemicity of hepatitis A has changed from high to intermediate [10]. In this context, various studies have evaluated the incidence, seroprevalence, risk factors, and vaccination status for hepatitis A [3,4,11-15], and have reported high incidence rates among individuals who are 20-39 years old. Nevertheless, it is likely that recent changes in age cohorts, vaccination status, lifestyle, and overseas travel have influenced the incidence of hepatitis A.
- The present study revealed that hepatitis A did not exhibit an increasing age effect, as the age-specific incidence was highest among young adults who were 20-39 years old. This result is consistent with the reported age-specific seroprevalence rate in Korea [11,12], as the high seroprevalence in individuals <10 years of age is related to vaccination that was introduced in 1997 [4], and the high seroprevalence in individuals >40 years of age is related to childhood infections that occurred before Korea’s rapid socioeconomic development and public sanitation improvements [11-13]. Therefore, hepatitis A occurred most frequently in the 20-39-year-old group, who exhibited a low seroprevalence rate. However, the incidence rapidly increased after the age of 18-20 years, which is when most individuals start college or develop an active social life. These adults were likely infected by HAV during overseas travel to endemic areas, after consuming shellfish, or at catered social events [4,16-19]. In addition, the incidence rates of hepatitis A were highest in 2009 for the 1978-1980 male birth cohort and the 1981-1983 female birth cohort. Although a difference of three years was found between males and females, the patterns confirm that the highest incidence was observed among individuals who were in their mid-20s.
- Analyzing hepatitis A incidence rates per 100,000 population, a gradually increasing pattern was seen from 2002 to 2007, followed by a sudden increase in 2008 and 2009. The cause of this spike in incidence is thought to be cases of sporadic occurrences in many regions, as observed in 2008 in the Gwangju region by Seo et al. [10] and an increase in laboratory diagnoses due to the rapid growth of awareness of hepatitis A.
- We observed a high incidence of HAV among 0-2-year-old children; although hepatitis A is generally mild and self-limiting among children, some cases involving complications have been reported [20,21]. In addition, some cases have required liver transplantation [22,23], which indicates that symptomatic and/or severe hepatitis A is possible among children. Furthermore, awareness among parents and pediatricians (based on the hepatitis A explosion during the late 2000s) has likely increased the number of children who have undergone diagnostic testing [24]. Thus, the increased incidence of hepatitis A among very young children may be related to the increased awareness and testing of patients with non-specific symptoms in that age group.
- A period effect was observed from 2008 to 2010, and the incidence of hepatitis A subsequently decreased after that point. This effect was likely related to a vaccination program that was introduced in Korea in 1997 [4]. Furthermore, the Korea Centers for Disease Control and Prevention started a national childhood vaccination program in May 2015 [3], which will likely help reduce the future incidence of hepatitis A.
- Variable birth cohort effects were observed, with high incidence rates observed in the pre-1930 cohorts and the 1980-1999 cohorts (20-39 years old at onset). Furthermore, the incidence of hepatitis A for the post-1978 cohorts increased continuously, although rapid decreases were observed in the post-2000 cohorts. Several studies of immunoglobulin G anti-HAV seropositivity have shown U-patterns with specific susceptible age groups. The birth years of such groups reported in previous studies have ranged approximately from 1970 to 2000, making those findings consistent with those of our study [11-13]. Various factors have likely influenced these effects. For example, these dramatic changes are closely related to the economic development of Korea, which was relatively weak until 1970, until which point Korea was characterized by poor public health, sanitation, and individual hygiene [10]. Thus, cohorts from this period likely had anti-HAV antibodies because they were exposed to HAV during their childhood [12]. In contrast, subsequent socioeconomic and sanitation improvements likely delayed HAV exposure in the post-1980 cohorts [11,13]. Nevertheless, a risk of exposure is still present, as Korean public sanitation has not yet improved to the level of advanced countries that have low HAV endemicity [10].
- The changes in the Korean endemicity of hepatitis A are likely a good example for developing countries, as hepatitis A is exhibiting a transition from high endemicity to intermediate endemicity in several Asian countries (e.g., China, India, and Malaysia) and Middle Eastern countries (e.g., Iran, Iraq, the United Arab Emirates, and Lebanon) [25-27]. Furthermore, hepatitis A in the Middle East may spread rapidly, given the poor hygiene, unclean water, and dense populations in Syrian refugee camps [27]. Moreover, hepatitis A vaccination is not currently sufficient in the Middle East, despite hepatitis A vaccination being included in several national immunization programs (e.g., in Bahrain, Cyprus, Iraq, Israel, Saudi Arabia, and Qatar) [27]. Therefore, since Korea has undergone rapid economic development, the patterns of change for hepatitis A in Korea may help predict future patterns in these countries.
- This study has several limitations. First, we were not able to determine the incidence of asymptomatic infections [10], as many children have subclinical symptoms of hepatitis A, and it is possible that we underestimated the incidence of hepatitis A in this age group. Second, we only evaluated data from an 11-year period, which is relatively short. However, hepatitis A is typically an acute disease that is cured within six weeks [2], and this study period was likely sufficient for evaluating the incidence and changing patterns of hepatitis A. Third, the health insurance data using our study were secondary data based on diagnostic codes used for insurance claims. Therefore, our findings may have been slightly different from those that would have been obtained using a gold-standard test for the disease based on laboratory identification of the immunoglobulin M anti-HAV antibody. Despite these limitations, our findings provide a good model of the changing epidemiological patterns of hepatitis A, which are based on Korean socioeconomic growth patterns that are currently reflected in Middle Eastern and Asian countries. Our results may help predict high incidence patterns that could occur in developing countries. If these countries are not properly prepared, outbreaks of hepatitis A may pose a significant threat to the health of the population and the national economy. Therefore, measures are needed to address the higher incidence rates of hepatitis A among vulnerable populations, and we recommend that governments in developing countries focus on improving sanitation (e.g., creating a purified water supply) and implementing national immunization programs to address future epidemiological changes in hepatitis A.
DISCUSSION
ACKNOWLEDGEMENTS
Figure 1.Age-standardized hepatitis A incidence rates per 100,000 population according to sex in Korea.


Figure 2.Trends in hepatitis A incidence rates per 100,000 population according to age and period in Korea. (A) Overall population, (B) male, and (C) female.
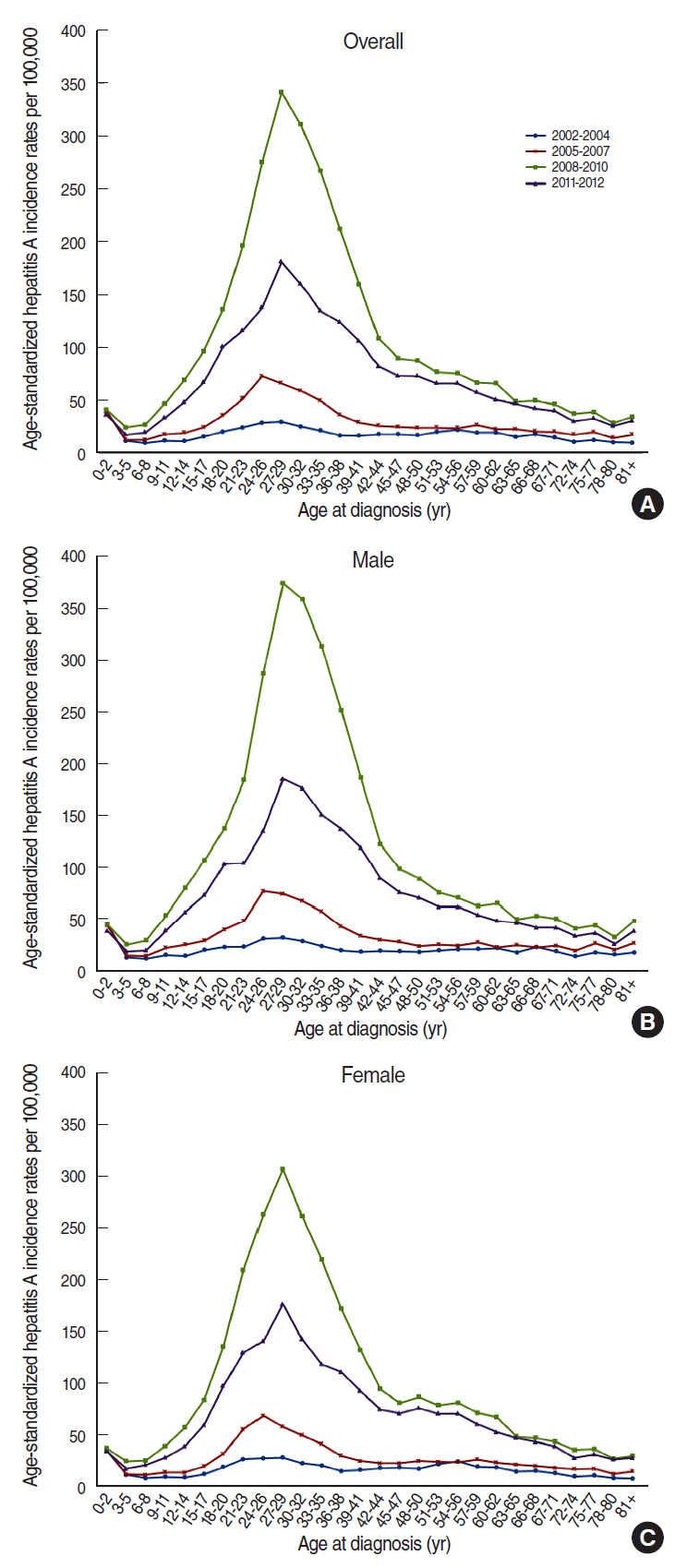

Figure 3.Age-period-cohort analysis of hepatitis A incidence in Korea. (A) Age effect, (B) period effect, and (C) cohort effect.


Table 1.Distribution of hepatitis A incidence according to year and sex in Korea, 2002-2012
Table 2.Age-standardized hepatitis A incidence rates per 100,000 population according to birth year and sex in Korea, 2002-2012
Table 3.Model fitness for age-period-cohort analyses of hepatitis A incidence rates according to sex in Korea, 2002-2012
- 1. Jung YM, Park SJ, Kim JS, Jang JH, Lee SH, Kim JW, et al. Atypical manifestations of hepatitis A infection: a prospective, multicenter study in Korea. J Med Virol 2010;82:1318-1326.ArticlePubMed
- 2. Jeong SH, Lee HS. Hepatitis A: clinical manifestations and management. Intervirology 2010;53:15-19.ArticlePubMed
- 3. Moon S, Han JH, Bae GR, Cho E, Kim B. Hepatitis A in Korea from 2011 to 2013: current epidemiologic status and regional distribution. J Korean Med Sci 2016;31:67-72.ArticlePubMedPMC
- 4. Seo JY, Choi BY, Ki M, Jang HL, Park HS, Son HJ, et al. Risk factors for acute hepatitis A infection in Korea in 2007 and 2009: a case-control study. J Korean Med Sci 2013;28:908-914.ArticlePubMedPMC
- 5. Choi HK, Song YG, Kim CO, Shin SY, Chin BS, Han SH, et al. Clinical features of re-emerging hepatitis A: an analysis of patients hospitalized during an urban epidemic in Korea. Yonsei Med J 2011;52:686-691.ArticlePubMedPMC
- 6. Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 2010;28:6653-6657.ArticlePubMed
- 7. Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect 2004;132:1005-1022.ArticlePubMedPMC
- 8. Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol 2005;34:600-609.ArticlePubMed
- 9. Yang Y, Sam SW, Wenjiang JF, Kenneth CL. The intrinsic estimator for age-period-cohort analysis: what it is and how to use it. Am J Sociol 2008;113:1697-1736.Article
- 10. Seo JY, Seo JH, Kim MH, Ki M, Park HS, Choi BY. Pattern of hepatitis a incidence according to area characteristics using national health insurance data. J Prev Med Public Health 2012;45:164-173.ArticlePubMedPMCPDF
- 11. Chung SJ, Kim TY, Kim SM, Roh M, Yu MY, Lee JH, et al. Changes in the seroprevalence of IgG anti-hepatitis A virus between 2001 and 2013: experience at a single center in Korea. Clin Mol Hepatol 2014;20:162-167.ArticlePubMedPMC
- 12. Lee H, Cho HK, Kim JH, Kim KH. Seroepidemiology of hepatitis A in Korea: changes over the past 30 years. J Korean Med Sci 2011;26:791-796.ArticlePubMedPMC
- 13. Cho SE, Kim Y. Seroepidemiology of hepatitis a in South Korea: a nationwide study by the Eone Reference Laboratory. J Epidemiol 2013;23:270-274.ArticlePubMedPMC
- 14. Song YJ, Lim J, Park WS, Sohn H, Lee MS, Shin DH, et al. Seropositivity among Korean young adults approximately 2 years after a single-dose vaccination against hepatitis A virus. PLoS One 2015;10:e0142297.ArticlePubMedPMC
- 15. Heo JY, Choe KW, Yoon CG, Jeong HW, Kim WJ, Cheong HJ. Vaccination policy in Korean armed forces: current status and future challenge. J Korean Med Sci 2015;30:353-359.ArticlePubMedPMC
- 16. Franco E, Giambi C, Ialacci R, Coppola RC, Zanetti AR. Risk groups for hepatitis A virus infection. Vaccine 2003;21:2224-2233.ArticlePubMed
- 17. Tosti ME, Spada E, Romanò L, Zanetti A, Mele A; SEIEVA collaborating group. Acute hepatitis A in Italy: incidence, risk factors and preventive measures. J Viral Hepat 2008;15 Suppl 2:26-32.ArticlePubMed
- 18. Wu D, Guo CY. Epidemiology and prevention of hepatitis A in travelers. J Travel Med 2013;20:394-399.ArticlePubMed
- 19. Askling HH, Rombo L, Andersson Y, Martin S, Ekdahl K. Hepatitis A risk in travelers. J Travel Med 2009;16:233-238.ArticlePubMed
- 20. Kumar KJ, Kumar HC, Manjunath VG, Anitha C, Mamatha S. Hepatitis A in children- clinical course, complications and laboratory profile. Indian J Pediatr 2014;81:15-19.ArticlePubMed
- 21. Yeung LT, Roberts EA. Current issues in the management of paediatric viral hepatitis. Liver Int 2010;30:5-18.ArticlePubMed
- 22. Thapa R, Mallick D, Biswas B, Ghosh A. Childhood hepatitis A virus infection complicated by Bell’s palsy. Clin Pediatr (Phila) 2009;48:427-428.ArticlePubMed
- 23. Shanmugam NP, Al-Lawati T, Kelgeri C, Rela M. Auxiliary liver transplantation for acute liver failure. Indian Pediatr 2016;53:67-69.ArticlePubMedPDF
- 24. Kong KA, Yoon SH, Cho SJ, Kim HW, Kim KH. Public acceptance and willingness to hepatitis a vaccination in children aged 7-18 years in Republic of Korea. J Korean Med Sci 2014;29:1528-1535.ArticlePubMedPMC
- 25. Cui F, Hadler SC, Zheng H, Wang F, Zhenhua W, Yuansheng H, et al. Hepatitis A surveillance and vaccine use in China from 1990 through 2007. J Epidemiol 2009;19:189-195.ArticlePubMedPMC
- 26. Yusoff FA, Rahman RA, May LH, Budart SB, Sulaiman LH. Investigation of hepatitis A outbreak in district of Manjung, Perak, Malaysia, October 2012. Western Pac Surveill Response J 2015;6:27-31.ArticlePubMedPMC
- 27. Melhem NM, Jaffa M, Zaatari M, Awada H, Salibi NE, Ramia S. The changing pattern of hepatitis A in Lebanese adults. Int J Infect Dis 2015;30:87-90.ArticlePubMed
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Hepatitis A vaccination
Li Zhang
Human Vaccines & Immunotherapeutics.2020; 16(7): 1565. CrossRef - Causes and countermeasures for repeated outbreaks of hepatitis A among adults in Korea
Moran Ki, Hyunjin Son, Bo Youl Choi
Epidemiology and Health.2019; 41: e2019038. CrossRef - The unrealized potential: cohort effects and age-period-cohort analysis
Jongho Heo, Sun-Young Jeon, Chang-Mo Oh, Jongnam Hwang, Juhwan Oh, Youngtae Cho
Epidemiology and Health.2017; 39: e2017056. CrossRef - Viral Hepatitis in South Korea
Stella C Pak, Yaseen Alastal, Zubair Khan, Umar Darr
Euroasian Journal of Hepato-Gastroenterology.2017; 7(2): 163. CrossRef

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite




