Articles
- Page Path
- HOME > Epidemiol Health > Volume 44; 2022 > Article
-
Systematic Review
The prevalence of Q fever in the Eastern Mediterranean region: a systematic review and meta-analysis -
Mozhgan Ahmadinezhad1,2
 , Leila Mounesan2
, Leila Mounesan2 , Amin Doosti-Irani3
, Amin Doosti-Irani3 , Manijeh Yousefi Behzadi2,4
, Manijeh Yousefi Behzadi2,4
-
Epidemiol Health 2022;44:e2022097.
DOI: https://doi.org/10.4178/epih.e2022097
Published online: October 28, 2022
1Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
2Department of Epidemiology and Biostatics, Research Centre for Emerging and Reemerging Infectious Diseases, Pasteur Institute of Iran, Tehran, Iran
3Department of Epidemiology, School of Public Health and Research Center for Health Sciences, Hamadan University of Medical Sciences, Hamadan, Iran
4National Reference Laboratory of Plague, Tularemia and Q Fever, Research Centre for Emerging and Reemerging Infectious Diseases, Pasteur Institute of Iran, Akanlu, KabudarAhang, Hamadan, Iran
- Correspondence: Manijeh Yousefi Behzadi Department of Epidemiology and Biostatics, Research Centre for Emerging and Reemerging Infectious Diseases, Pasteur Institute of Iran, P.O. Box, Tehran 1316943551, Iran E-mail: yousefibehzadim@yahoo.fr
© 2022, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
OBJECTIVES
- Q fever, caused by the bacterium, is a major zoonotic disease around the world. This disease is common in the Eastern Mediterranean region; therefore, we conducted the first systematic review and meta-analysis on its prevalence in humans, animals, and ticks in the Eastern Mediterranean region.
-
METHODS
- Major Iranian and international databases were searched from 2000 to 2021. We extracted the prevalence of Q fever in blood samples from animals and milk samples from animals, ticks, and humans as the main outcome. We reported the prevalence of seropositivity and molecular positivity as point estimates and 95% confidence intervals (CIs).
-
RESULTS
- In this review, 112 papers were identified. The overall seroprevalence of Q fever was 22.4% (95% CI, 19.8 to 25.1). The pooled prevalence of Q fever in ticks was 17.5% (95% CI, -1.3 to 36.4). The prevalence was 25.5% (95% CI, 16.1 to 34.9) in humans. The prevalence of Q fever in animal blood samples from goats, sheep, camels, cattle, cats, dogs, horses, and buffalo were 28.1%, 25.1%, 25.0%, 20.1%, 9.8%, 8.4%, 6.5%, and 6.3%, respectively. Furthermore, the prevalence of Q fever in milk samples of animals was higher in cattle (20.3%) than in sheep (20.0%), goats (16.4%), and camels (3.3%).
-
CONCLUSIONS
- Coxiella burnetii infections are common in humans and in a wide range of animal species, but they are still not recognized in many countries in the Eastern Mediterranean region, thus presenting a significant threat to human and animal health in the region.
- Emerging and re-emerging infectious diseases are recognized worldwide as a threat to public health. More than half of these are zoonotic diseases [1] that are often transmitted to humans through direct contact with animals, their carcasses, and animal-derived products [2].
- Q fever is a zoonotic disease [3] that leads to public health problems worldwide and is especially common in developing countries. This disease is caused by Coxiella burnetii and infects humans, wild animals, and domestic animals [4]. The C. burnetii bacterium grows in the environment and enters the body of ticks and mites [5,6]. The infection then enters the body of domestic ruminants (mainly cattle, sheep, and goats), which constitute the most important reservoir of C. burnetii [7,8].
- Q fever is mostly asymptomatic in animals, except in cases where it causes miscarriage, stillbirth, and infertility [9]. Humans are also susceptible to this disease. The main route of transmission of Q fever to humans is through inhalation of contaminated aerosols or dust containing C. burnetii [10]. Additionally, human contact with feces, urine, embryos, and placentas of infected animals can transmit the disease [11,12]. However, humans can also contract C. burnetii through biting and consuming dairy products [13]. In humans, the clinical manifestations of C. burnetii infection include acute or chronic syndromes [14]. The most common form of the disease is similar to the flu. The clinical manifestations include fever, headache, coughing, atypical pneumonia, hepatitis, myalgia, arthralgia, cardiac involvement, skin rash, and neurological signs, and 2% of patients with the acute form of the disease are hospitalized [15]. This disease has imposed substantial costs on patients and health systems [16].
- Many countries in the world are exposed to Q fever. However, in some regions, the prevalence of this disease is different; for instance, in low-income countries such as the Eastern Mediterranean region (EMR), this disease has become a health problem [17- 19]. Review studies conducted in Iran [20], Pakistan [21], and Tunisia [22] have studied the prevalence of Q fever in animals, humans, and dairy products. However, there has not yet been a systematic review and meta-analysis that compiles and reviews all relevant studies in this field in the EMR.
- Despite variation in certain health and disease indices, countries of the EMR region have similar cultural, economic, and medical characteristics. Furthermore, addressing the prevalence of Q fever would be useful for designing effective intervention strategies to control the disease. Therefore, the purpose of this systematic review and meta-analysis was to conduct a comprehensive epidemiological study of Q fever in the EMR.
INTRODUCTION
- Literature search strategy
- Initially, we searched PubMed, ISI Web of Science, and Scopus databases as major international databases, and Magiran and SID for Persian-language articles. These national databases cover Iranian scientific journals.
- The keywords that we used for our search were “Q fever,” “Coxiella burnetii,” “query fever,” and “Pakistan,” “Afghanistan,” “Bahrain,” “Djibouti,” “Egypt,” “Iran,” “Iraq,” “Jordan,” “Kuwait,” “Lebanon,” ”Libya,” “Morocco,” “Oman,” “Qatar,” “Somalia,” “Saudi Arabia,” “Syria,” “Sudan,” “Tunisia,” “United Arab Emirates” and “Yemen” in English sources. For the Iranian databases (Magiran and SID), we used both English and Persian keywords.
- We searched the databases for articles that reported the prevalence of Q fever in humans, ticks, and mites, and in milk and blood samples from animals such as cattle, sheep, goats, camels, buffalo, and horses, cats, and dogs in the EMR. We started the search in the year 2000 because an increased interest has been seen in Q fever research in the EMR since this year. All articles reporting C. burnetii prevalence in humans or animals by any serological or molecular method were included in the study. The researchers used enzyme-linked immunosorbent assays and indirect immunofluorescence assays to diagnose Q fever through serological methods and polymerase chain reaction as a molecular method.
- Eligibility criteria and study selection
- We first performed the screening using titles first then abstracts and full texts of the studies were reviewed independently by 2 authors (MA and MYB). Any disagreement between these 2 authors was resolved by a third author (ADI). The authors of this meta-analysis reviewed the studies based on established inclusion criteria.
- The inclusion criteria included studies measuring the prevalence of Q fever in ticks and mites, humans, and animal specimens (including cattle, sheep, goats, camels, buffalo, dogs, cats, and horses). Studies conducted in the countries of the EMR region from 2000 to 2021 were included in our meta-analysis.
- The exclusion criteria included (1) letters, books, editorials, reports, and reviews; (2) studies where the place of sample collection and its origin were not known; (3) studies that did not clearly state the sample size and positive cases; and (4) studies in countries other than the EMR.
- Data extraction
- We used a pre-designed template to extract data from the imported articles. The extracted data included disease/pathogen, year, country, design, species/occupation, number of animals/humans/samples tested, method of diagnosis, study outcomes, and the first author of each study. Data extraction was conducted independently by the same 2 review authors (MA and MYB) who conducted the study selection.
- Quality assessment
- The quality of papers was assessed using the Newcastle-Ottawa quality assessment scale (NOS) designed for human observational studies [23]. The NOS consists of 3 domains: the selection of study groups, comparability of groups, and description of exposure and outcome.
- This quality assessment tool includes 6-8 items and star scores for each study in each domain. All items have 1 star except the comparability domain (the maximum score based on stars for the comparability domain is 2). The total score of each article was calculated. Then, all the selected studies were categorized as high (5-7), medium (4-3), or low quality (< 3). Two authors (MA and ADI) reviewed the articles separately. The opinion of the third author was used to address any issues of disagreement.
- Statistical analysis
- We estimated the prevalence of Q fever with 95% confidence intervals (CIs) by subgroups of species and country. Statistical heterogeneity was explored using the I2 statistic. We adopted a random-effects model to estimate the prevalence of Q fever and performed subgroup analyses by the species of data collection, country, year of publication, and occupation. To explore the main factors influencing prevalence estimates and sources of heterogeneity, we conducted a meta-regression analysis for species, country variables, year of publication, and occupation. Publication bias was assessed using the Egger and Begg tests, with p-value < 0.05 indicating significant bias. The analysis was performed using Stata version 16 (Stata Corp., College Station, TX, USA).
- Ethics statement
- Ethical approval was not sought because this study was based on published articles and no human or animal intervention was performed.
MATERIALS AND METHODS
- Study characteristics
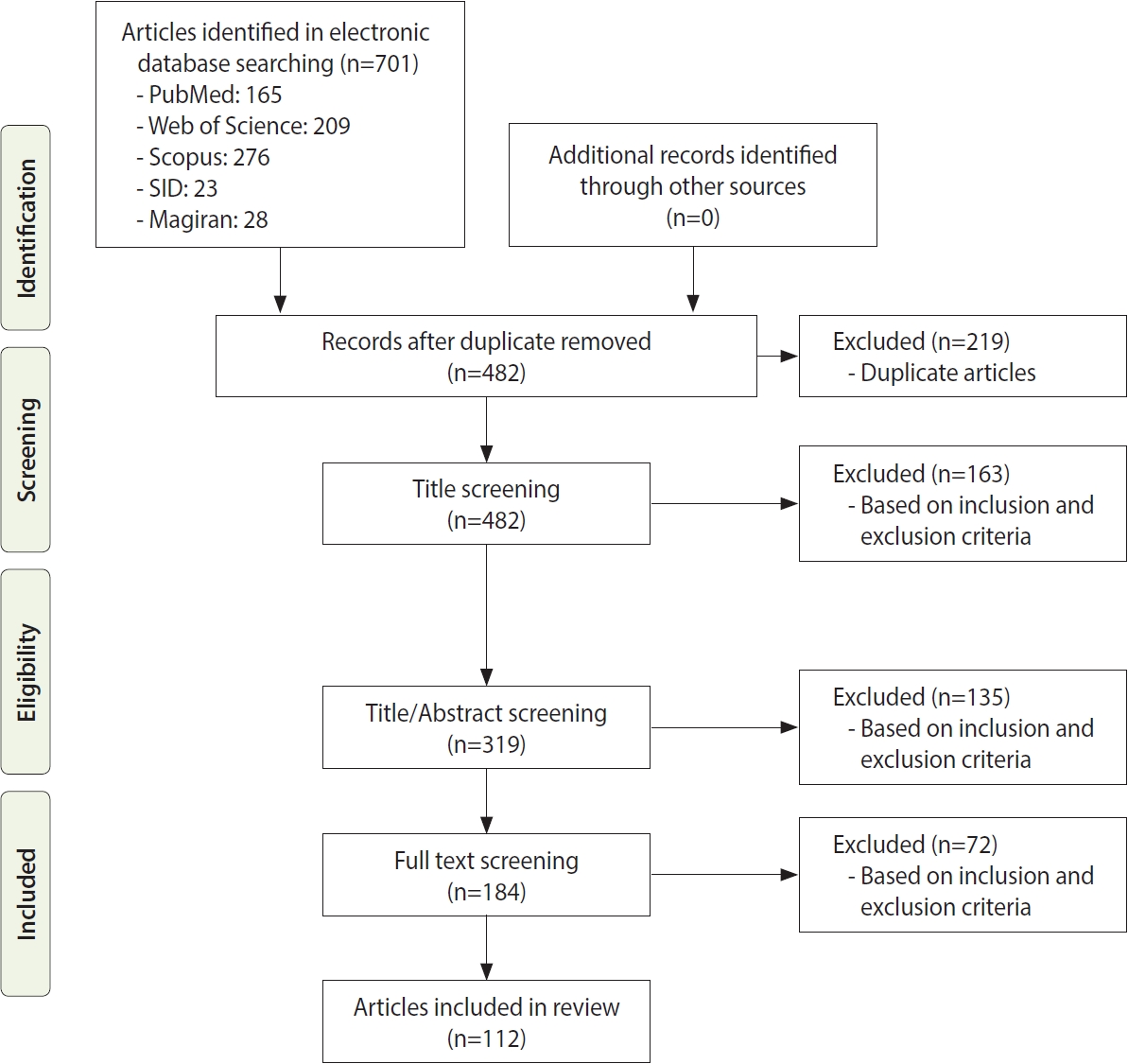
- Figure 1 shows the search strategy and the algorithm of study selection were shown. According to the keywords and Medical Subject Heading terms, 701 studies were identified, of which 163 articles were extracted from the PubMed database, 209 articles from Web of Science, 276 articles from Scopus, 23 articles from SID, and 28 articles from Magiran. Out of these articles, 219 were duplicates that were excluded in the first stage. After identifying relevant studies and considering the inclusion and exclusion criteria, 163 studies, 135 studies, and 72 studies were excluded after screening their titles, abstracts, and full texts, respectively. The studies were reviewed based on the 4-step process of Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2009 (Figure 1), including identifying articles, screening, reviewing the criteria for accepting articles, and determining the articles that entered the meta-analysis process. Finally, 112 articles were included in the final analysis; their information is given in Supplementary Material 1. Of these studies, 66 were performed in Iran; 14 in Egypt; 5 each in Saudi Arabia and Tunisia; 4 each in Pakistan, Iraq, and Afghanistan; 3 each in the United Arab Emirates and Jordan; and 1 each in Sudan, Oman, Somalia, and Lebanon. All articles that were finally included in this study were cross-sectional. We performed a quality assessment for human studies only because the NOS tool is defined for human studies. Nineteen of the 35 (54.2%) included studies had NOS scores of 5-7, indicating that they had high levels of quality, and 12 of the 35 (34.3%) included studies had NOS scores of 3-4, indicating moderate levels of quality (Supplementary Material 1). There was a limited number 4 of the 35 (11.4%) of low-quality studies.
- Publication bias
- The results of statistical testing for publication bias, including Begg and Egger tests, for Q fever, were statistically significant for animal milk and blood samples (all p< 0.001). In addition, the result of the Begg test for Q fever in humans was statistically significant (p= 0.01), whereas the result of the Egger test for osteopenia of the lumbar spine was not statistically significant (p= 0.59). Furthermore, the results of the Egger and Begg tests for Q fever in humans were not statistically significant (p= 0.42 and 0.08, respectively). These results confirmed the presence of publication bias.
- The pooled prevalence of Q fever
- Of the 112 studies that were included in our meta-analysis, most of the studies were conducted on humans and in Iran. The overall prevalence of Q fever in the EMR in humans, animal species, mites and ticks, and milk samples was 22.4% (95% CI, 19.8 to 25.1) and the total heterogeneity was I2 = 98.7 (Table 1). According to Table 1, most studies were from 2010 onwards, and studies of Q fever have been conducted in most EMR countries since 2010. Due to the heterogeneity of the selected studies, a random-effects model was used to combine the reported results of the studies.
- Occupation
- In human studies, the subjects’ occupations were included in the studies; most studies were conducted among high-risk subjects (butchers, farmers, and veterinarians). However, a limited number of studies have examined other occupations, such as vaccinators, the military, and laboratory occupations. The prevalence estimates of Q fever in the general population, butchers, veterinarians, farmers, herders, military, and other categories were 28.1% (95% CI, 18.0 to 38.3), 31.9% (95% CI, 15.9 to 48.0), 15.6% (95% CI, 2.0 to 29.1), 22.5% (95% CI, 10.5 to 34.4), 62.4% (95% CI, 18.4 to 88.0), 20.3% (95% CI, 2.3 to 38.4), 21.0% (95% CI, 0.0 to 42.5), respectively. The heterogeneity of all occupations was above 86% (Table 1).
- Country
- The meta-analysis of studies evaluating Afghanistan (n = 4) showed that the prevalence of Q fever was 34.4% (95% CI, 0.0 to 76.8) (Table 1) with a high level of heterogeneity (99.9 %). The prevalence estimates of Q fever in Egypt, Iran, Iraq, Jordan, Lebanon, Oman, Pakistan, Saudi Arabia, Somalia, Sudan, Tunisia, and the United Arab Emirates were 25.6% (95% CI, 19.0 to 32.3), 20.8% (95% CI, 17.3 to 24.3), 22.2% (95% CI, 0.0 to 44.4), 44.4% (95% CI, 29.6 to 59.1), 20.2% (95% CI, 9.7 to 30.6), 30.0% (95% CI, 0.0 to 71.9), 22.3% (95% CI, 12.4 to 32.2), 20.4% (95% CI, 12.5 to 28.3), 59.1% (95% CI, 52.8 to 65.3), 23.7% (95% CI, 19.8 to 27.6), 15.8% (95% CI, 1.3 to 30.4), and 27.0% (95% CI, 3.5 to 50.5), , respectively. The heterogeneity of all countries was also above 96% (Table 1).
- Species
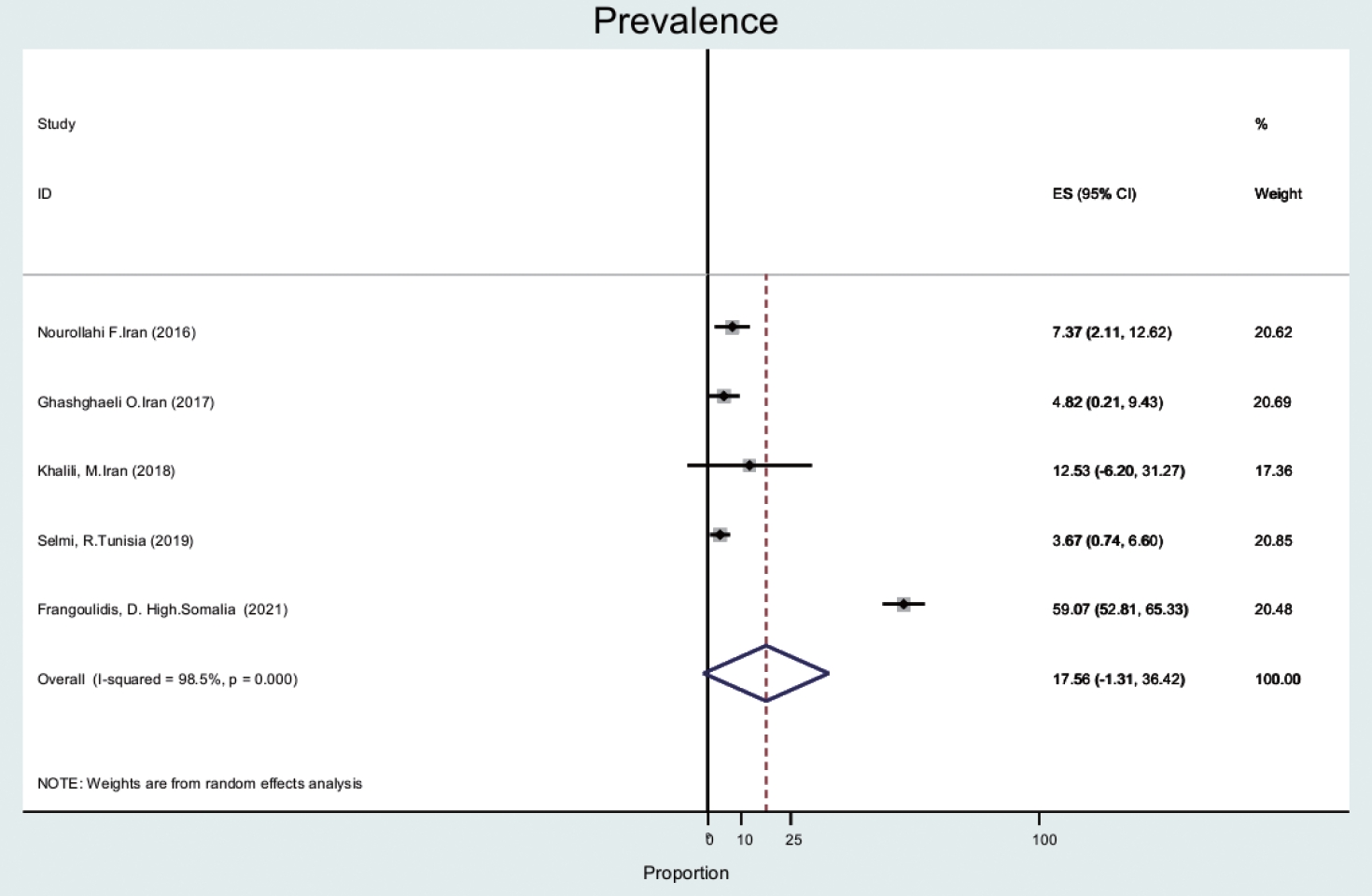
- The prevalence of Q fever in ticks (n= 5) was 17.5% (95% CI, -1.3 to 36.4), with a high level of heterogeneity (98.5%) (Figure 2). Ticks and mites were collected from animals such as camels, dogs, and small ruminants [24-26].
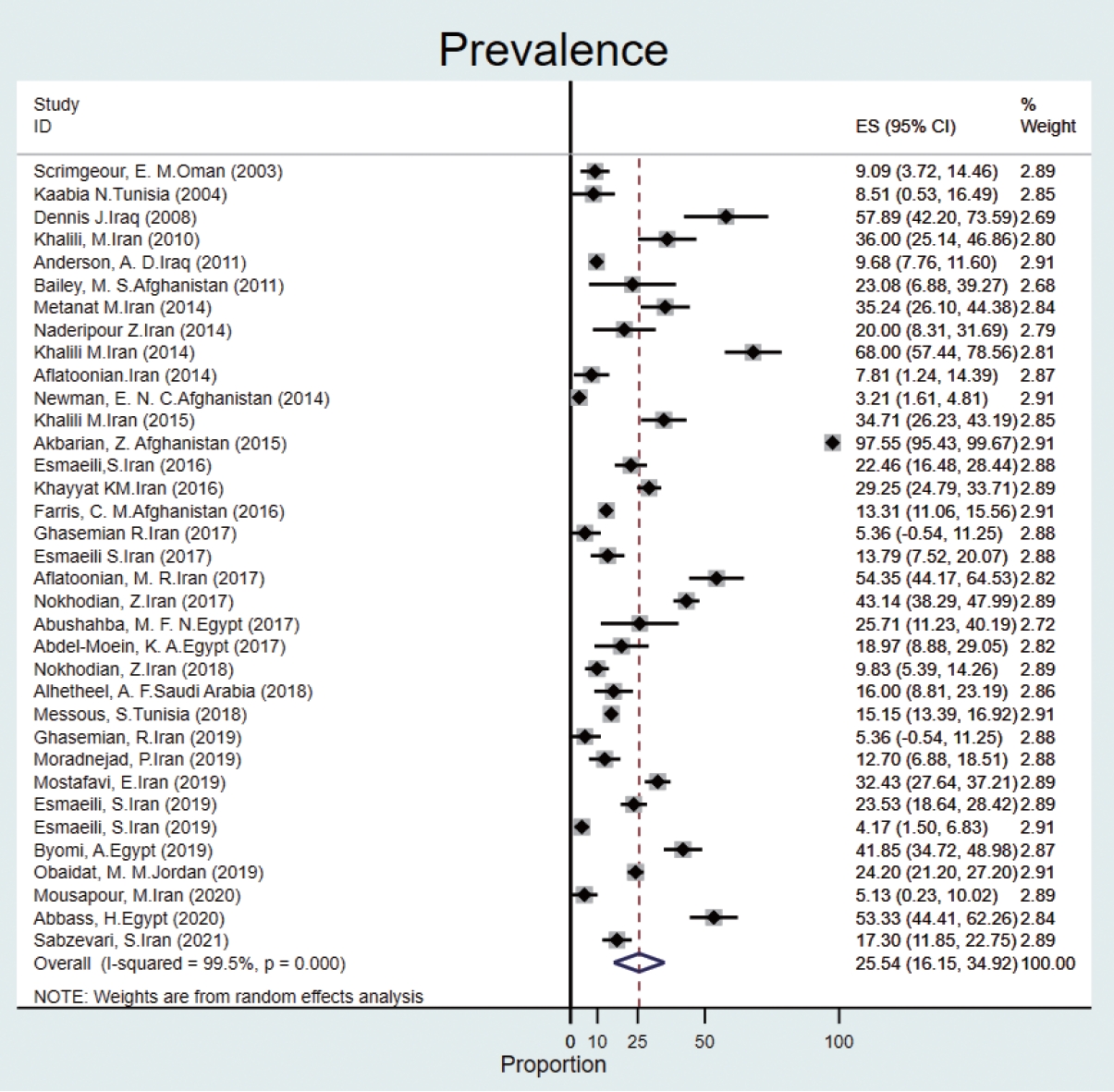
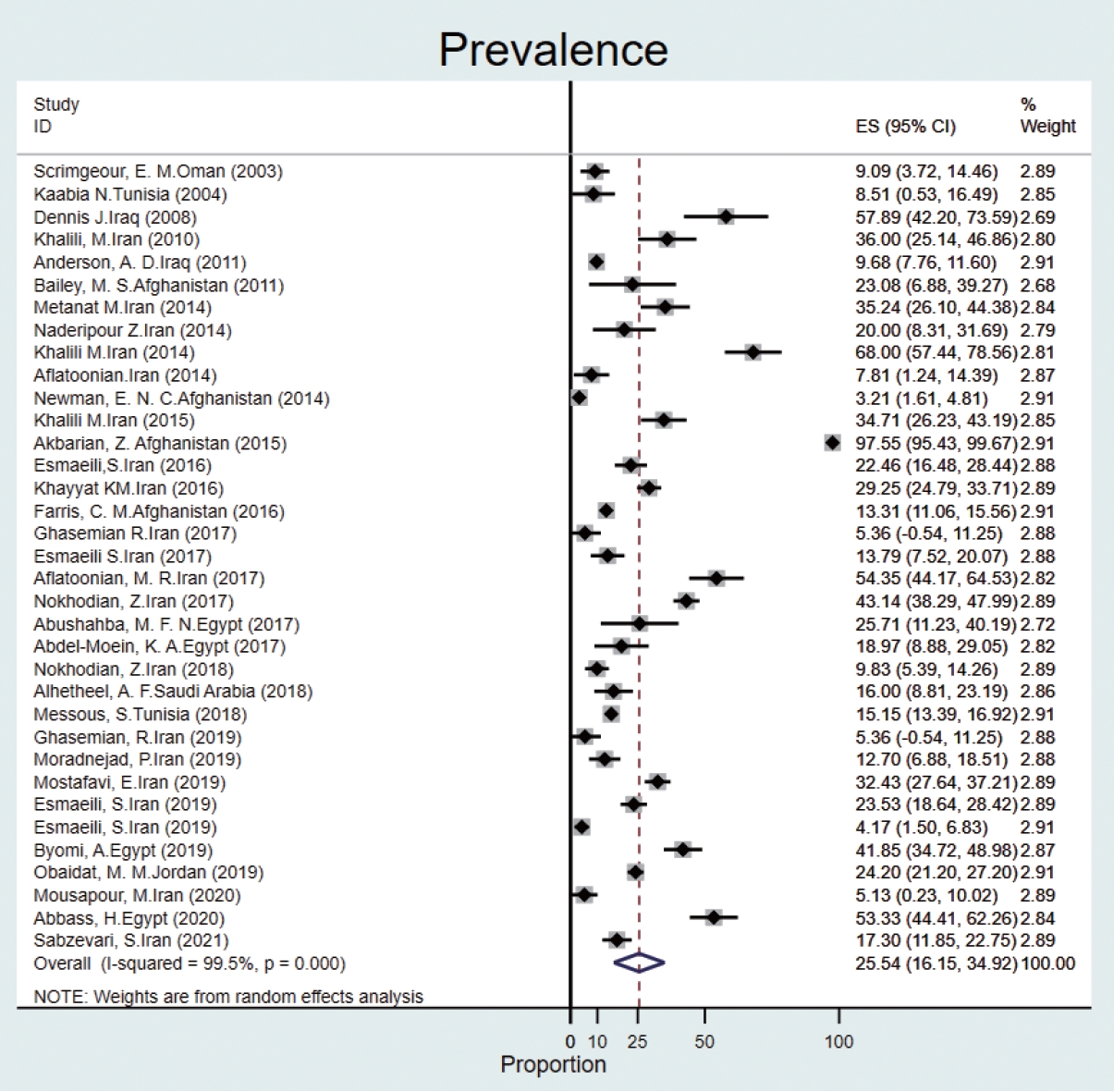
- The prevalence of Q fever in human blood samples was estimated to be 25.5% (95% CI, 16.1 to 34.9), with high heterogeneity (I2 = 99.5%) (Figure 3).
- In animals, milk and blood samples were collected from different species. Eighty out of 92 studies were related to goats, sheep, camels, and cattle, with wide variations in prevalence.
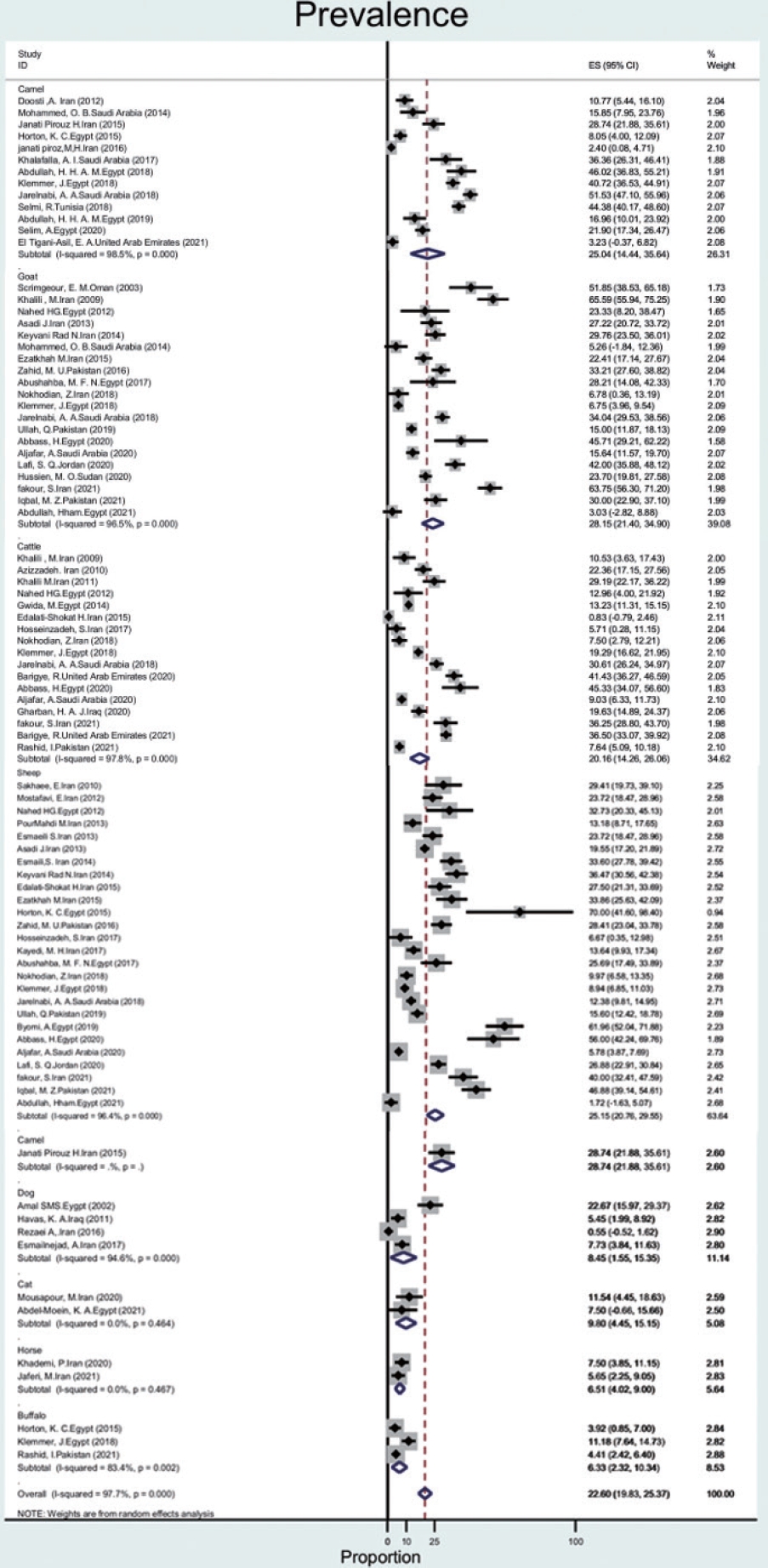
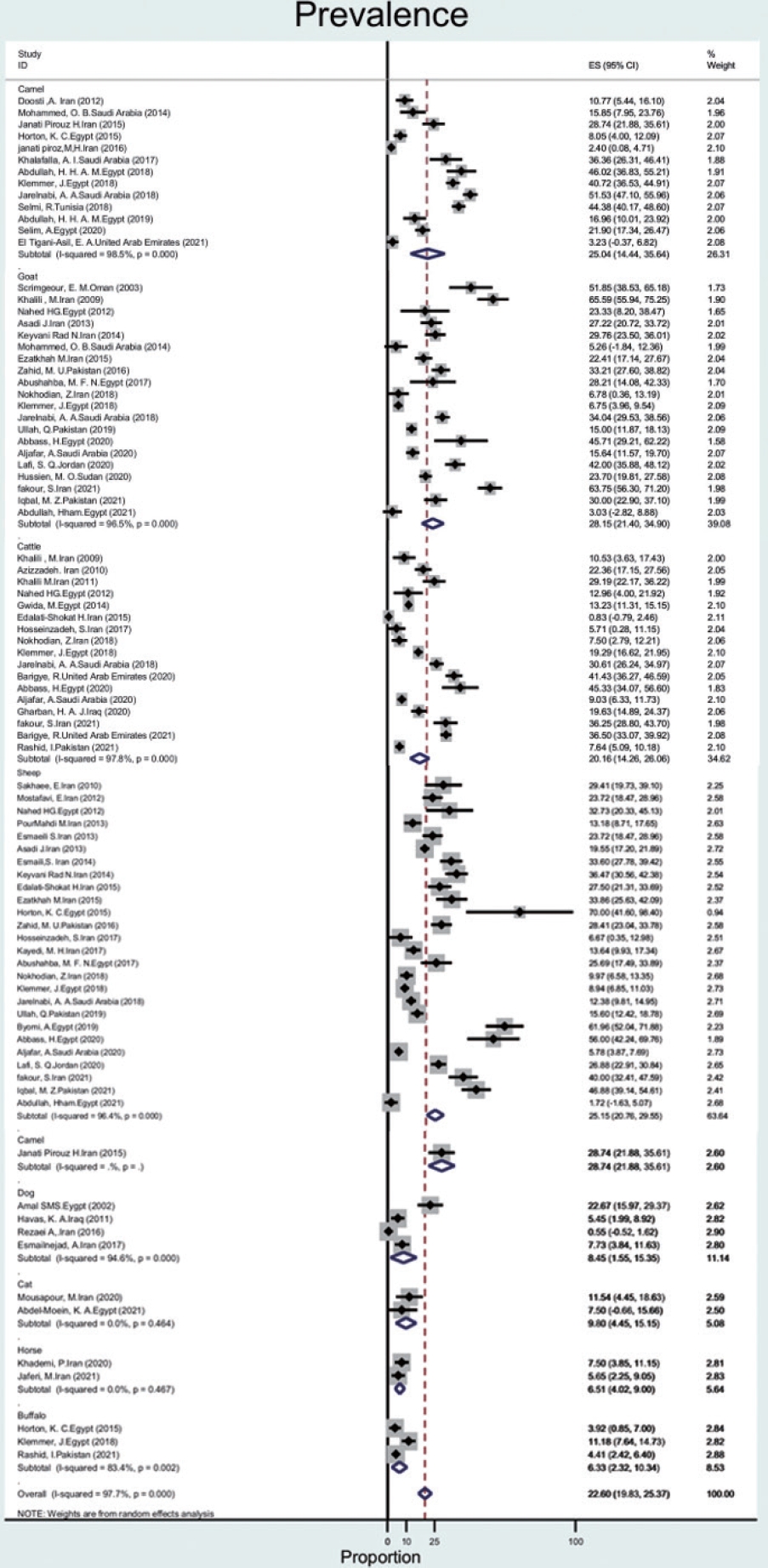
- The prevalence of Q fever in blood samples from cattle (n= 17) was 20.1% (95% CI, 14.2 to 26.0), with a high level of heterogeneity (97.8%). In goats, sheep, camels, dogs, cats, buffalo, and horses, the prevalence of Q fever was 28.1% (95% CI, 21.4 to 34.9), 25.1% (95% CI, 20.7 to 29.5), 25.0% (95% CI, 14.4 to 35.6), 8.4% (95% CI, 1.5 to 15.3), 9.8% (95% CI, 4.4 to 15.1), 6.3% (95% CI, 2.3 to 10.3), 6.5% (95% CI, 4.0 to 9.0), with heterogeneity of 96.5%, 96.4%, 98.5%,94.6%, not applicable, 83.4%, and not applicable, respectively (Figure 4).
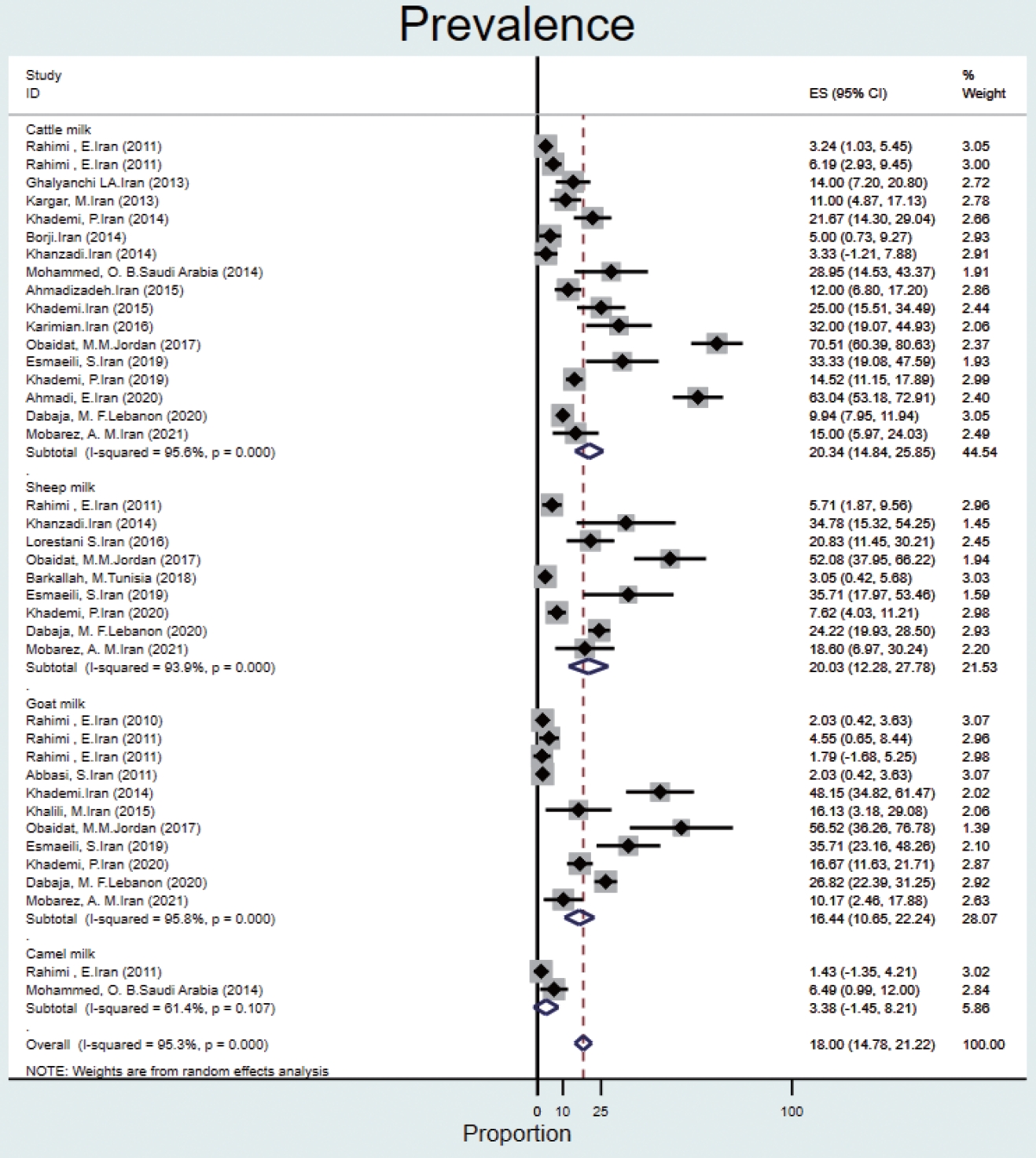
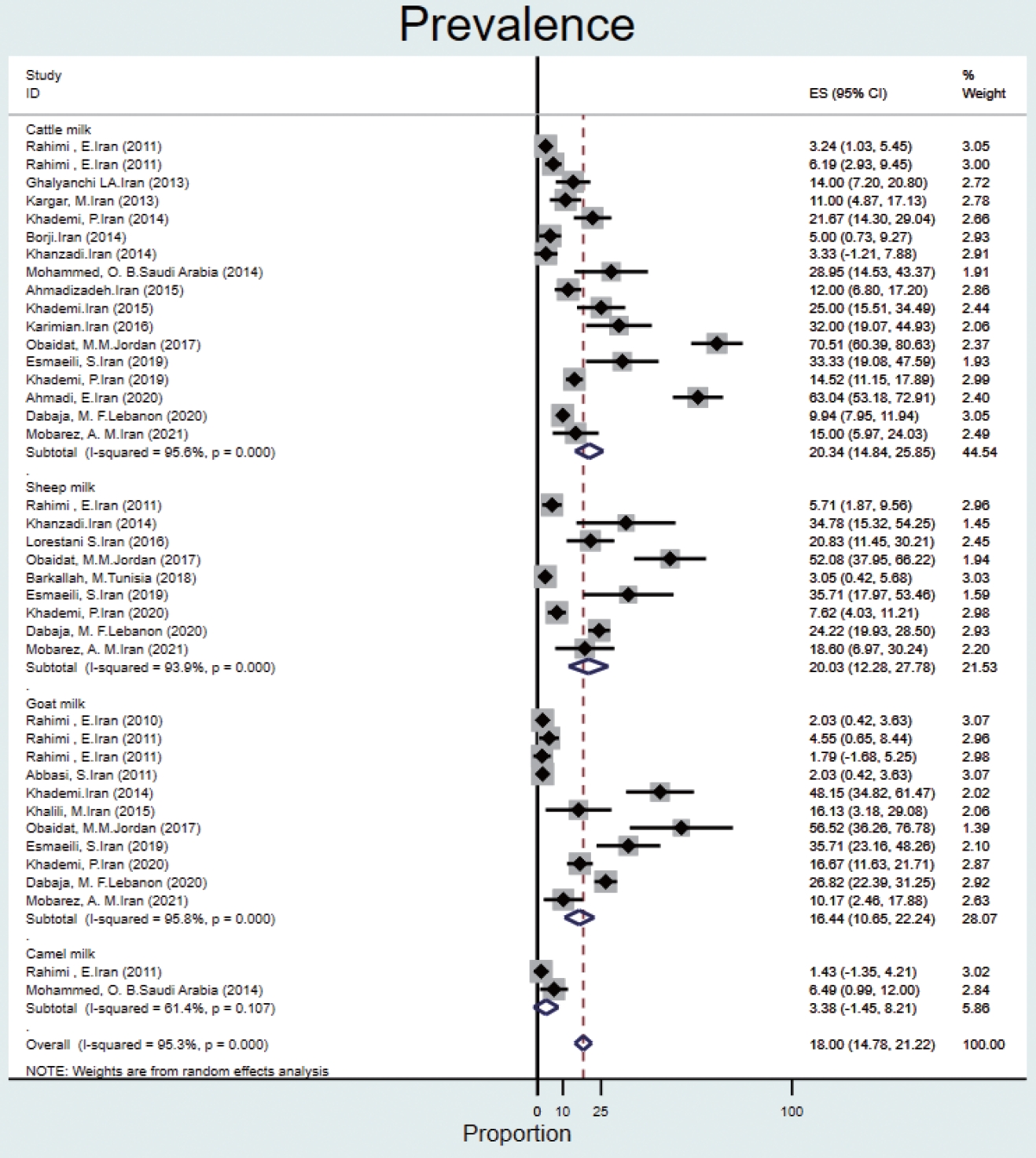
- The prevalence of Q fever in milk samples from cattle (n= 17) was 20.3% (95% CI, 14.8 to 25.8), and high heterogeneity was observed (95.6%). In sheep, goat, and camel milk samples, the prevalence of Q fever was 20.0% (95% CI, 12.2 to 27.7), 16.4% (95% CI, 10.6 to 22.2), 3.3% (95% CI, -1.4 to 8.2), and the heterogeneity was 93.9%, 95.8%, and 61.4%, respectively (Figure 5).
- Meta-regression
- The heterogeneity across studies was particularly high when studies were evaluated overall. For this reason, we performed subgroup analyses and meta-regression. In the subgroup analyses, the I2 statistics ranged from 61.4% to 99.5% (Table 1). The meta-regression results showed that species significantly affected the estimation of point prevalence (p= 0.04) (Table 2). That is, studies on certain species showed an excessively high prevalence of Q fever. However, this result does not fully explain the high level of heterogeneity observed (Table 2).
RESULTS
Overall and subgroup
Ticks
Humans
Animal blood
Animal milk
- Based on data from 112 studies in the EMR during 2000-2021, we found that Q fever is relatively common among humans, animals, and ticks, with a pooled estimate of 22.4%. The prevalence of Q fever among ticks was 17.5% (95% CI, -1.3 to 36.4). The prevalence of Q fever in blood samples from humans was 25.5% (95% CI, 16.1 to 34.9), that for cattle was 20.1% (95% CI, 14.2 to 26.0), that for goats was 28.1% (95% CI, 21.4 to 34.9), that for sheep was 25.1% (95% CI, 20.7 to 29.5), that for camels was 25.0% (95% CI, 14.4 to 35.6), that for dogs was 8.4% (95% CI, 1.5 to 15.3), that for cats was 9.8% (95% CI, 4.4 to 15.1), that for buffalo was 6.3% (95% CI, 2.3 to 10.3) and that for horses was 6.5% (95% CI, 4.0 to 9.0). The prevalence of Q fever in milk samples from cattle was 20.3% (95% CI, 14.8 to 25.8), that for sheep was 20.0% (95% CI, 12.2 to 27.7), that for goats was 16.4% (95% CI, 10.6 to 22.2), and that for camels was 3.3% (95% CI, -1.4 to 8.2).
- However, data on Q fever prevalence were only reported for 14 countries of the region, and there is still a dearth of data across the region, which might be a result of limited resources. Most of the studies were conducted in Iran, Egypt, and Saudi Arabia, and more than half of the studies were carried out in the past 5 years. The results of a review on hotspots of Q fever research from 1990 to 2019, showed that Iran had a total number of publications of 46 (ranked 15th) and was a productive country, with 11 articles in 2019 [27].
- The overall seroprevalence in humans was reported in a wide range of countries in the EMR. The results of a systematic review in Iran from 2000 to 2015 revealed that seroprevalence of Q fever in humans was different in various locations; for instance, immunoglobulin G antibodies were reported in 68% and 27.83% of Iranians at high risk in the southeast and western regions, respectively [28]. In our study, the prevalence of Q fever in humans was about 26%, which is similar to the prevalence in the western region of Iran. In the EMR, the highest prevalence of antibodies was seen in Afghanistan (2015), where serological evidence of exposure to 97% of humans (from 204 blood samples) were seropositive for C. burnetii, and the lowest (5.12%) was reported in Iran [29,30]. Furthermore, in Iran, 14 epidemiological studies on the distribution of Q fever in humans were conducted between 2016 and 2021. In Egypt, 2 studies (2019-2020) reported that the prevalence of Q fever in humans was 41.8% and 53.3%, respectively [11-25]. The results of studies in Saudi Arabia (2018), Oman (2003), and Tunisia (2004) showed that C. burnetii antibodies were detected in 16.0%, 9.8%, and 8.5% of individuals, respectively. In Iraq, the overall percentage of people who had seroconverted to Q fever was 10% (from 909 serum samples). Furthermore, the prevalence of acute Q fever was reported in 5 studies (4 from Iran and 1 from Afghanistan), with a range of 5.3% to 35.2% [31-34]. Therefore, the prevalence of Q fever varies from country to country and even from city to city. Humans can become infected by exposure, most often through inhalation of airborne particles, animal feces, or the release of C. burnetii into the environment through feces [35]. Q fever prevalence studies showed evidence of C. burnetii prevalence ranging from 15.6% to 62.0% in high-risk populations, including veterinarians, butchers, and farmers [36- 38]. Therefore, this disease can be classified as an occupational disease [39]. Furthermore, the frequency of livestock breeding and the infrastructure of microbiological diagnostic tests, specific epidemiological care, and awareness of the disease affect the spread or control of this disease [35]. Although the prevalence of Q fever may be underestimated, as the symptoms are non-specific and similar to those of other infectious diseases [32], several studies on human antibodies to C. burnetii antigens show that Q fever is a major challenge in many countries [40].
- Ticks, as main vectors, play an important role in Q fever transmission. The results of our study were slightly different from those of a meta-analysis that was conducted on rodent parasites in the Middle East. In our study, tick prevalence was 18%, compared to 25% in the Middle East [41]. This small difference may be explained by a different context. A systematic study conducted in Europe [42] found the prevalence of Q fever in ticks was 5%, which was different from the prevalence in our study because the prevalence of Q fever in ticks in European countries is lower than in the EMR and this indicates the difference in abundance of species in 2 different regions. This reason may explain the difference in prevalence.
- The prevalence of C. burnetii in specimens of hard-bodied ticks was only reported in Iran, Somalia, and Tunisia. The results showed that 140 of 237 (59.1%) tick samples in Somalia were positive. In Iran and Tunisia, tick samples were positive in a range of 3% to 17% [43-46] and the tick genera included Rhipicephalus and Hyalomma. In our study, tick samples were obtained from animals including dogs, camels, and small ruminants.
- In the region, goats (28%), sheep (25%), and camels (25%) seem to pose the greatest risk for human infection. The results of our study are consistent with a study in Iran that investigated the prevalence of Q fever in animals in 2018. In this study, the total prevalence of Q fever in animals such as cattle, sheep, and goats in Iran was found to be 27%, of which the highest prevalence was 33% for goats. In our study, the prevalence of Q fever in goats (28%) than that of other animals [28]. Furthermore, in line with our results, in the United States, goats (42%) and sheep (16%) appear to pose a greater risk of human infection than cattle or wild animals [3]. The C. burnetii seroprevalence in camel herds can reach more than 60% in Egypt, Saudi Arabia, and Sudan [47]. Although the prevalence of C. burnetii in ruminants was over 20%, it was less than 10% in dogs, cats, camels, and horses. Therefore, this disease poses a major risk for the contamination of ruminants.
- In milk samples, the highest prevalence was reported in goats (48%) and sheep (21%) in Iran [48,49]. In a study in the United States [50], the prevalence of Q fever in bulk tank milk of dairy herds was ≥ 94%. The high prevalence of C. burnetii in dairy herds can reflect the lack of effective vaccines and treatment protocols for infected animals [50]. In a meta-analysis conducted in Iran [51], the total prevalence of C. burnetii was 15.09% in cattle milk, 7.80% in goat milk, and 3.79% in sheep milk. This disease has become a special challenge in a system with mixed herds of cattle, sheep, and goats, and it is considered one of the important problems of livestock production all over the world [52].
- According to One Health goals, monitoring and surveillance of Q fever in livestock, wildlife, and humans is crucial in identifying and controlling outbreaks [53]. Furthermore, controlling external parasites such as ticks should be considered to reduce their adverse effects [54].
- The most important strength of the present study is the comprehensive review of all databases, the independent review of articles by 2 researchers, and the performance of meta-regression and subgroup analyses to obtain more accurate information. The present study aimed to address the limitations of other systematic reviews and meta-analyses in this field by conducting a comprehensive review of different sources over a long period, with meta-regression and subgroup analyses. However, the wide variety of sampling, testing, and recording methods in different studies and the relatively long period of 21 years may reduce the comparability of the findings.
DISCUSSION
- Our study findings demonstrate that Q fever is a public health issue in the EMR. For an effective zoonotic disease surveillance program in the region, additional research on human and animal species in areas where there is a lack of knowledge about the distribution of Q fever is necessary, in addition to interventions for prevention and control.
CONCLUSION
SUPPLEMENTARY MATERIALS
Supplementary Material 1.
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
-
FUNDING
None.
-
AUTHOR CONTRIBUTIONS
Conceptualization: Yousefi Behzadi M. Data curation: Ahmadinezhad M, Yousefi Behzadi M, Doosti-Irani A. Formal analysis: Ahmadinezhad M, Doosti-Irani A, Mounesan L. Funding acquisition: None. Methodology: Ahmadinezhad M, Doosti-Irani A. Project administration: Yousefi Behzadi M. Visualization: Ahmadinezhad M, Yousefi Behzadi M. Writing – original draft: Ahmadinezhad M, Mounesan L. Writing – review & editing: Ahmadinezhad M, Yousefi Behzadi M, Mounesan L, Doosti-Irani A.
NOTES
ACKNOWLEDGEMENTS

| Covariate | Meta-regression coefficient |
95% CI |
p-value | |
|---|---|---|---|---|
| LL | UL | |||
| Year of publication | 0.1 | -4.7 | 5.0 | 0.95 |
| Country | -0.1 | -0.8 | 0.6 | 0.75 |
| Species | 0.1 | 0.0 | 0.2 | 0.04 |
| Occupation | -0.2 | -1.5 | 1.0 | 0.70 |
- 1. Buliva E, Elhakim M, Tran Minh NN, Elkholy A, Mala P, Abubakar A, et al. Emerging and reemerging diseases in the World Health Organization (WHO) Eastern Mediterranean regionprogress, challenges, and WHO initiatives. Front Public Health 2017;5:276.ArticlePubMedPMC
- 2. Mostafavi E, Ghasemian A, Abdinasir A, Nematollahi Mahani SA, Rawaf S, Salehi Vaziri M, et al. Emerging and re-emerging infectious diseases in the WHO Eastern Mediterranean region, 2001-2018. Int J Health Policy Manag 2021;11:1286-1300.ArticlePubMedPMCPDF
- 3. McQuiston JH, Childs JE. Q fever in humans and animals in the United States. Vector Borne Zoonotic Dis 2002;2:179-191.ArticlePubMed
- 4. Angelakis E, Raoult D. Emergence of Q fever. Iran J Public Health 2011;40:1-18.
- 5. Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Bassene H, Molez JF, et al. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis 2010;4:e654.ArticlePubMedPMC
- 6. Tissot-Dupont H, Raoult D. Q fever. Infect Dis Clin North Am 2008;22:505-514.ArticlePubMed
- 7. Pouquet M, Bareille N, Guatteo R, Moret L, Beaudeau F. Coxiella burnetii infection in humans: to what extent do cattle in infected areas free from small ruminants play a role? Epidemiol Infect 2020;148:e232.ArticlePubMedPMC
- 8. Porter SR, Czaplicki G, Mainil J, Guattéo R, Saegerman C. Q fever: current state of knowledge and perspectives of research of a neglected zoonosis. Int J Microbiol 2011;2011:248418.ArticlePubMedPMCPDF
- 9. Raoult D, Marrie T, Mege J. Natural history and pathophysiology of Q fever. Lancet Infect Dis 2005;5:219-226.ArticlePubMed
- 10. Gürtler L, Bauerfeind U, Blümel J, Burger R, Drosten C, Gröner A, et al. Coxiella burnetii - pathogenic agent of Q (Query) fever. Transfus Med Hemother 2014;41:60-72.ArticlePubMedPMCPDF
- 11. Hersom M, Irsik M, Thrift T. Biosecurity and biological risk management for livestock enterprises; 2008 [cited 2023 Feb 10]. Available from: https://edis.ifas.ufl.edu/publication/AN194.
- 12. Honarmand H. Q fever: an old but still a poorly understood disease. Interdiscip Perspect Infect Dis 2012;2012:131932.ArticlePubMedPMCPDF
- 13. Abdali F, Hosseinzadeh S, Berizi E, Shams S. Prevalence of Coxiella burnetii in unpasteurized dairy products using nested PCR assay. Iran J Microbiol 2018;10:220-226.PubMedPMC
- 14. Raoult D. Reemergence of Q fever after 11 September 2001. Clin Infect Dis 2009;48:558-559.ArticlePubMed
- 15. Marrie TJ. Q fever pneumonia. Infect Dis Clin North Am 2010;24:27-41.ArticlePubMed
- 16. Porter SR, Czaplicki G, Mainil J, Guattéo R, Saegerman C. Q fever: current state of knowledge and perspectives of research of a neglected zoonosis. Int J Microbiol 2011;2011:248418.ArticlePubMedPMCPDF
- 17. Abdullah HH, Hussein HA, El-Razik KA, Barakat AM, Soliman YA. Q fever: a neglected disease of camels in Giza and Cairo Provinces, Egypt. Vet World 2019;12:1945-1950.ArticlePubMedPMCPDF
- 18. Barigye R, Hassan NA, Abdalla Alfaki IM, Barongo MB, Mohamed ME, Mohteshamuddin K. Seroprevalence of Coxiella burnetii in a dairy cattle herd from the Al Ain region, United Arab Emirates. Trop Anim Health Prod 2021;53:112.ArticlePubMedPDF
- 19. Aljafar A, Salem M, Housawi F, Zaghawa A, Hegazy Y. Seroprevalence and risk factors of Q-fever (C. burnetii infection) among ruminants reared in the eastern region of the Kingdom of Saudi Arabia. Trop Anim Health Prod 2020;52:2631-2638.ArticlePubMedPDF
- 20. Behzadi MY, Mostafavi E, Rohani M, Mohamadi A, Ahmadinezhad M, Moazzezy N, et al. A review on important zoonotic bacterial tick-borne diseases in the Eastern Mediterranean region. J Arthropod Borne Dis 2021;15:265-277.PubMedPMC
- 21. Ghafar A, Abbas T, Rehman A, Sandhu ZU, Cabezas-Cruz A, Jabbar A. Systematic review of ticks and tick-borne pathogens of small ruminants in Pakistan. Pathogens 2020;9:937.ArticlePubMedPMC
- 22. Kaabia N, Letaief A. Q fever in Tunisia. Pathol Biol (Paris) 2009;57:439-443 (French).PubMed
- 23. Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011.
- 24. Frangoulidis D, Kahlhofer C, Said AS, Osman AY, Chitimia-Dobler L, Shuaib YA. High prevalence and new genotype of Coxiella burnetii in ticks infesting camels in Somalia. Pathogens 2021;10:741.ArticlePubMedPMC
- 25. Selmi R, Mamlouk A, Ben Yahia H, Abdelaali H, Ben Said M, Sellami K, et al. Coxiella burnetii in Tunisian dromedary camels (Camelus dromedarius): seroprevalence, associated risk factors and seasonal dynamics. Acta Trop 2018;188:234-239.ArticlePubMed
- 26. Ghashghaei O, Fard SR, Khalili M, Sharifi H. A survey of ixodid ticks feeding on cattle and molecular detectionof Coxiella burnetii from ticks in Southeast Iran. Turk J Vet Anim Sci 2017;41:46-50.Article
- 27. Farooq M, Khan AU, El-Adawy H, Mertens-Scholz K, Khan I, Neubauer H, et al. Research trends and hotspots of Q fever research: a bibliometric analysis 1990-2019. Biomed Res Int 2022;2022:9324471.ArticlePubMedPMCPDF
- 28. Nokhodian Z, Feizi A, Ataei B, Hoseini SG, Mostafavi E. Epidemiology of Q fever in Iran: a systematic review and meta-analysis for estimating serological and molecular prevalence. J Res Med Sci 2017;22:121.ArticlePubMedPMC
- 29. Khademi P, Jaydari A, Esmaeili Koutamehr M. Genomic detection of Coxiella burnetii in cattle milk samples by nested-PCR method, Iran. Iran J Med Microbiol 2015;9:69-72 (Persian).
- 30. Esmaeili S, Mohabati Mobarez A, Khalili M, Mostafavi E. High prevalence and risk factors of Coxiella burnetii in milk of dairy animals with a history of abortion in Iran. Comp Immunol Microbiol Infect Dis 2019;63:127-130.ArticlePubMed
- 31. Scrimgeour EM, Al-Ismaily SI, Rolain JM, Al-Dhahry SH, ElKhatim HS, Raoult D. Q fever in human and livestock populations in Oman. Ann N Y Acad Sci 2003;990:221-225.ArticlePubMed
- 32. Kaabia N, Rolain JM, Khalifa M, Ben Jazia E, Bahri F, Raoult D, et al. Serologic study of rickettsioses among acute febrile patients in central Tunisia. Ann N Y Acad Sci 2006;1078:176-179.ArticlePubMed
- 33. Faix DJ, Harrison DJ, Riddle MS, Vaughn AF, Yingst SL, Earhart K, et al. Outbreak of Q fever among US military in western Iraq, June-July 2005. Clin Infect Dis 2008;46:e65-e68.ArticlePubMed
- 34. Alhetheel AF, Binkhamis K, Somily A, Barry M, Shakoor Z. Screening for Q fever. A tertiary care hospital-based experience in central Saudi Arabia. Saudi Med J 2018;39:1195-1199.PubMedPMC
- 35. Epelboin L, Eldin C, Thill P, de Santi VP, Abboud P, Walter G, et al. Human Q fever on the Guiana shield and Brazil: recent findings and remaining questions. Curr Trop Med Rep 2021;8:173-182.ArticlePubMedPMCPDF
- 36. Sabzevari S, Shoraka H, Seyyedin M. Seroepidemiological survey of brucellosis and Q fever among high-risk occupations in northeast of Iran for first time. Iran J Microbiol 2021;13:325-336.ArticlePubMedPMCPDF
- 37. Esmaeili S, Mohabati Mobarez A, Khalili M, Mostafavi E, Moradnejad P. Genetic evidence of Coxiella burnetii infection in acute febrile illnesses in Iran. PLoS Negl Trop Dis 2019;13:e0007181.ArticlePubMedPMC
- 38. Abushahba MF, Abdelbaset AE, Rawy MS, Ahmed SO. Crosssectional study for determining the prevalence of Q fever in small ruminants and humans at El Minya Governorate, Egypt. BMC Res Notes 2017;10:538.ArticlePubMedPMCPDF
- 39. Abbass H, Selim SA, Sobhy MM, El-Mokhtar MA, Elhariri M, Abd-Elhafeez HH. High prevalence of Coxiella burnetii infection in humans and livestock in Assiut, Egypt: a serological and molecular survey. Vet World 2020;13:2578-2586.ArticlePubMedPMCPDF
- 40. Szymańska-Czerwińska M, Galińska EM, Niemczuk K, Knap JP. Prevalence of Coxiella burnetii infection in humans occupationally exposed to animals in Poland. Vector Borne Zoonotic Dis 2015;15:261-267.ArticlePubMedPMC
- 41. Islam MM, Farag E, Eltom K, Hassan MM, Bansal D, Schaffner F, et al. Rodent ectoparasites in the Middle East: a systematic review and meta-analysis. Pathogens 2021;10:139.ArticlePubMedPMC
- 42. Körner S, Makert GR, Ulbert S, Pfeffer M, Mertens-Scholz K. The prevalence of Coxiella burnetii in hard ticks in Europe and their role in Q fever transmission revisited-a systematic review. Front Vet Sci 2021;8:655715.ArticlePubMedPMC
- 43. Abdullah HH, El-Shanawany EE, Abdel-Shafy S, Abou-Zeina HA, Abdel-Rahman EH. Molecular and immunological characterization of Hyalomma dromedarii and Hyalomma excavatum (Acari: Ixodidae) vectors of Q fever in camels. Vet World 2018;11:1109-1119.ArticlePubMedPMCPDF
- 44. Esmaeili S, Bagheri Amiri F, Mokhayeri H, Kayedi MH, Maurin M, Rohani M, et al. Seroepidemiological study of Q fever, brucellosis and tularemia in butchers and slaughterhouses workers in Lorestan, western of Iran. Comp Immunol Microbiol Infect Dis 2019;66:101322.ArticlePubMed
- 45. Zahid MU, Hussain MH, Saqib M, Neubauer H, Abbas G, Khan I, et al. Seroprevalence of Q fever (Coxiellosis) in small ruminants of two districts in Punjab, Pakistan. Vector Borne Zoonotic Dis 2016;16:449-454.ArticlePubMed
- 46. Abdel-Moein KA, Hamza DA. The burden of Coxiella burnetii among aborted dairy animals in Egypt and its public health implications. Acta Trop 2017;166:92-95.ArticlePubMed
- 47. Devaux CA, Osman IO, Million M, Raoult D. Coxiella burnetii in dromedary camels (Camelus dromedarius): a possible threat for humans and livestock in North Africa and the near and Middle East? Front Vet Sci 2020;7:558481.ArticlePubMedPMC
- 48. Khademi P, Mahzounieh M, Koutamehr ME. Genomic detection of Coxiella burnetii (Q fever agent) in cattles milk samples in animal farms Bonab township. Iran. Iranian J Public Health 2014;43(Suppl 2):292.
- 49. Khademi P, Mahzounieh MR, Kahrizsangi AE, Shdravan E. Genomic detection of Coxiella burnetii in goat milk samples in animal farms Khorramabad Township, Iran. Pajhohandeh 2014;3:166-72 (Persian).
- 50. Kim SG, Kim EH, Lafferty CJ, Dubovi E. Coxiella burnetii in bulk tank milk samples, United States. Emerg Infect Dis 2005;11:619-621.ArticlePubMedPMC
- 51. Esmaeili S, Mohabati Mobarez A, Khalili M, Mostafavi E, Moradnejad P. Molecular prevalence of Coxiella burnetii in milk in Iran: a systematic review and meta-analysis. Trop Anim Health Prod 2019;51:1345-1355.ArticlePubMedPDF
- 52. Thornton PK. Livestock production: recent trends, future prospects. Philos Trans R Soc Lond B Biol Sci 2010;365:2853-2867.ArticlePubMedPMCPDF
- 53. Espí A, Del Cerro A, Oleaga Á, Rodríguez-Pérez M, López CM, Hurtado A, et al. One Health approach: an overview of Q fever in livestock, wildlife and humans in Asturias (Northwestern Spain). Animals (Basel) 2021;11:1395.ArticlePubMedPMC
- 54. Muhammad A, Bashir R, Mahmood M, Afzal MS, Simsek S, Awan UA, et al. Epidemiology of ectoparasites (ticks, lice, and mites) in the livestock of Pakistan: a review. Front Vet Sci 2021;8:780738.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Prevalence, Risk Factors, and Relationship between Reproductive Performance and the Presence of Antibodies against Coxiellosis in Dairy Farm Milk Tanks in the Northwest of Spain
Uxía Yáñez, Jacobo Álvarez, Cristina Pisón, Antía Acción, Juan J. Becerra, Antonio Jiménez, Philippe Gisbert, Pedro G. Herradón, Ana I. Peña, Alberto Prieto, José M. Díaz-Cao, Luis A. Quintela
Animals.2024; 14(3): 367. CrossRef - Molecular detection of Coxiella burnetii in tick and blood samples from small ruminants in northwest of Iran
Ahmad Enferadi Ghazanabad, Negin Esfandiari, Reza Najafi, Shahryar Mehrabi, Saeedeh Sarani, Peyman Khademi, Max Maurin
Experimental and Applied Acarology.2024; 92(3): 529. CrossRef - Global and regional seroprevalence of coxiellosis in small ruminants: A systematic review and meta‐analysis
Md Ahaduzzaman, Md Moktadir Billah Reza
Veterinary Medicine and Science.2024;[Epub] CrossRef - Two Cases of Acute Q Fever Diagnosed by mNGS and Literature Review
娇 罗
Advances in Clinical Medicine.2023; 13(07): 12005. CrossRef - Zoonotic diseases transmitted from the camels
Abdelmalik Ibrahim Khalafalla
Frontiers in Veterinary Science.2023;[Epub] CrossRef - Q fever and coxiellosis: implications for livestock and human health in the UK
Nick Wheelhouse, Richard Vazquez, Lorenzo Viora, Jo E. B. Halliday
Livestock.2023; 28(5): 221. CrossRef
- Figure
- Related articles
-
- Effectiveness of community-based interventions for older adults living alone: a systematic review and meta-analysis
- Dietary intake and cancer incidence in Korean adults: a systematic review and meta-analysis of observational studies
- The prevalence of functional disability and its impact on older adults in the ASEAN region: a systematic review and meta-analysis

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite