The Mysuru stUdies of Determinants of Health in Rural Adults (MUDHRA), India
Article information
Abstract
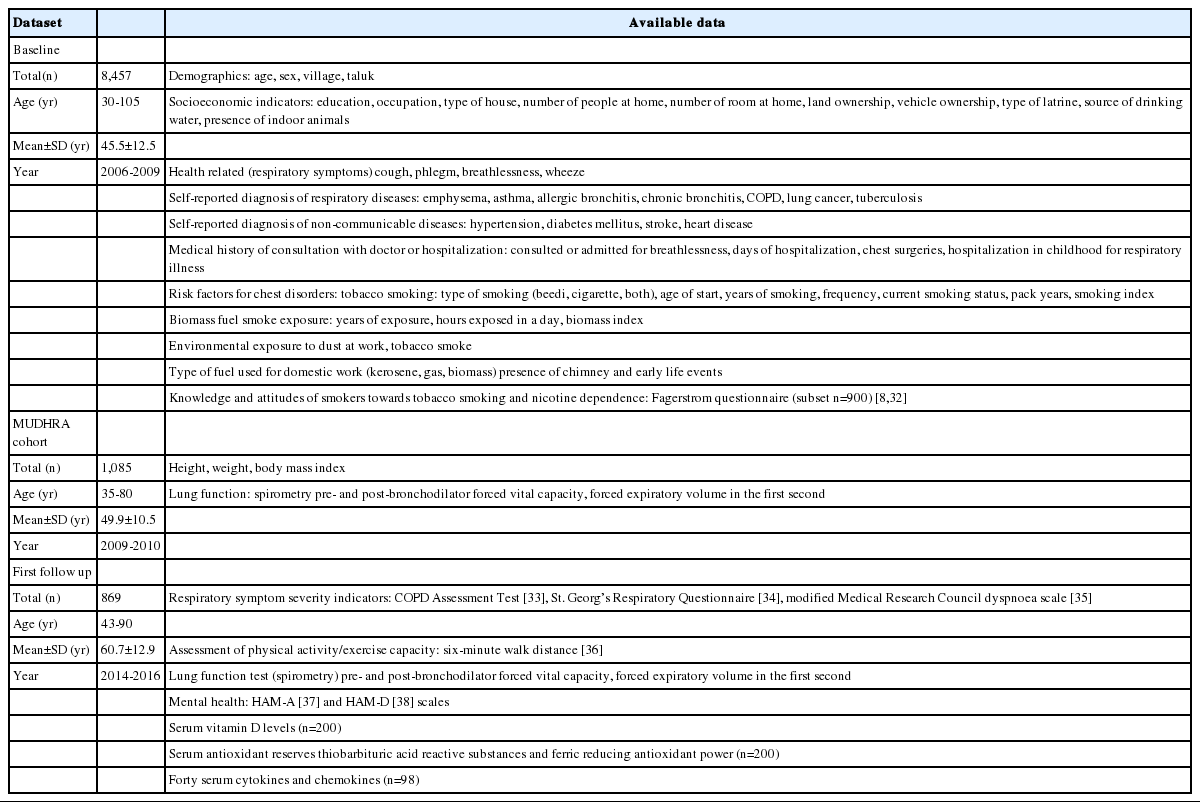
Between 2006 and 2010, in 16 randomly selected villages in rural areas of Mysore district, in south India, 8,457 subjects aged 30 and above were screened for symptoms of chronic respiratory disease. Of the 8,457 subjects, 1,692 were randomly invited for further evaluation of lung function and chronic obstructive pulmonary disease (COPD) by spirometry, and 1,085 of these subjects underwent lung function assessments for prevalent COPD and its risk factors. These 1,085 subjects, who were then aged between 35 and 80 years, constituted the Mysuru stUdies of Determinants of Health in Rural Adults (MUDHRA) cohort. Among other findings, threshold of biomass fuel smoke exposure suitable for use as a dichotomous risk factor for the diagnosis of chronic bronchitis was established, with a minimum biomass smoke exposure index of 60 found to be significantly associated with an elevated risk of developing chronic bronchitis. Five years later (between 2014 and 2016), 869 of the 1,085 participants were followed up with repeat lung function assessments for incident COPD and all-cause mortality. A subset of these participants (n=200) underwent blood tests for vitamin D levels, antioxidant activity, an assessment for anxiety and depression, and another subset (n=98) underwent a bioplex assay for 40 serum cytokines.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major cause of the global burden of morbidity and mortality and is projected to be the third leading cause of death by 2030 [1-3]. Of the more than 170 million COPD patients worldwide, more than 3 million people died in 2015. COPD accounts for 2.6% of global disability-adjusted life years. The burden of COPD is particularly high in rural areas of low- and middle-income countries, and it is the second leading cause of death in India [1,4,5] due to relatively high levels of smoking, ambient air pollution, exposure to biomass smoke, ozone, occupational particulate matter, and environmental tobacco smoke [4,6]. More than 3 billion people in the world are exposed to biomass smoke, compared to 1 billion smokers. We have shown previously that more than 90% of rural households used biomass fuels and that more than 50% of rural men smoked. Despite this, limited data exist on the prevalence of COPD and its burden in rural India [5].
In India, more than 70% of the population lives in rural areas [7]. The prevalence rates of COPD reported in population-based studies from rural India were not derived from standardised lung function assessments, but based on questionnaires [5,8-23]. Furthermore, in many studies, the participants were not randomly selected from the general population, which limits the generalizability of the findings. In those studies, the association of the dose of biomass smoke exposure with chronic respiratory disorders, particularly among non-smokers, was not examined. Those studies were primarily cross-sectional in design and were therefore limited to reporting prevalent COPD, and participants were not followed up to estimate the incidence of chronic respiratory disorders [5,8-23]. Therefore, the Mysuru stUdies of Determinants of Health of Rural Adults (MUDHRA) cohort was established to address some of these limitations, and specifically to examine the risk factors for prevalent and incident COPD among rural men and women in southern India.
COPD is characterized by local and systemic inflammation involving various chemokines and cytokines, and the pulmonary inflammation extends into the systemic circulation [24-28]. In Indian COPD patients, pulmonary and systemic inflammation is due to exposure to important risk factors, such as tobacco smoking (usually in men) or biomass smoke exposure (usually in women). Exposure to these risk factors leads to increased levels of pro-inflammatory cytokines and reduced levels of anti-inflammatory and homeostatic cytokines. The interaction of various cytokines and chemokines is insufficiently understood, especially in biomass smoke-related COPD, and preliminary data have shown that biomass smoke-related COPD is different from tobacco smoke-related COPD [29]. Some cytokines are associated with increased inflammation in COPD (interleukin [IL]-6 and tumour necrosis factor alpha [TNF-α]), the progression of disease (IL-2), or neutrophil recruitment (granulocyte-macrophage colony-stimulating factor, IL-8), whereas anti-inflammatory cytokines, such as IL-10, help to mitigate the inflammation. It is necessary to understand the cytokine signatures in COPD due to tobacco and biomass smoke, so a sub-sample of this cohort was evaluated to investigate differences in the cytokine signatures of tobacco smokerelated COPD and biomass smoke-related COPD.
STUDY PARTICIPANTS
Of the 7 taluks (sub-districts) in the district of Mysuru, 2 were randomly selected: rural Mysuru with 176 villages and Nanjangud with 131 villages. According to the 2001 Registrar General India census [7], each of these villages had 1,800-2,200 men and women above 30 years of age who were potentially eligible for recruitment. The sample size estimation indicated that we needed to screen at least 8,000 men and women aged above 30 years from these villages to obtain approximately 1,000 cases of symptomatic chronic chest disease. This estimation was based on an estimated COPD prevalence of 5% in this population, with 80% power and 10% standard error. Therefore, of the 307 villages, 8 villages from Mysuru and 8 villages from Nanjangud were randomly selected. Figure 1 shows a map of India illustrating the sampling sites from which the MUDHRA cohort was drawn.
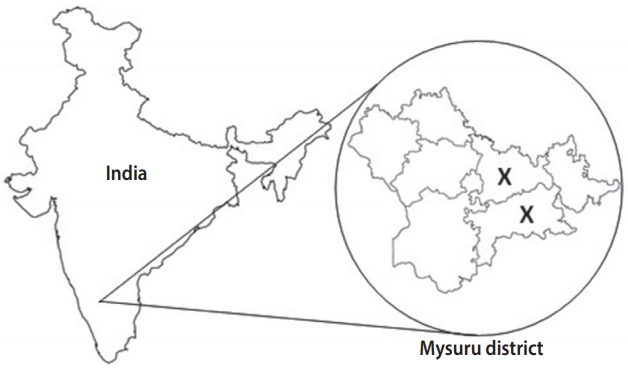
Map of India illustrating the site of sampling from which the Mysuru stUdies of Determinants of Health in Rural Adults (MUDHRA) cohort was set up (X depicts the 2 rural sub-districts selected for the study).
Trained field workers conducted a door-to-door survey of all households (n = 3,139) from these 16 villages to identify those above 30 years of age. Houses were visited on at least 3 separate occasions before being declared non-responders (n= 196). Potentially eligible members from each household underwent a standardised structured interview adopted from the Burden of Obstructive Lung Diseases study [30]. This questionnaire was used to obtain information about socio-demographic variables, respiratory symptoms, self-reported diagnosis of respiratory disease and other non-communicable diseases, medical history of consultations with a doctor or hospitalisation, and exposure to risk factors for chest disorders such as biomass fuel smoke and tobacco smoke.
In the first phase, a total of 8,457 subjects (women: n=3,953, 46.7%; men: n= 4,054, 53.3%) from 3,943 households were screened for symptoms of chronic respiratory diseases and the prevalence of chronic bronchitis. Data were also collected on socioeconomic status, education, occupation, tobacco and alcohol consumption, and biomass smoke exposure. Twenty percent (n= 1,692) of these subjects were randomly invited for further evaluation of lung function and COPD by spirometry. Among 1,692 subjects, 423 declined to participate (62 men and 361 women). More women and men refused to participate due to sociocultural issues. Of the 1,269 subjects who underwent spirometry, 1,085 satisfied the American Thoracic Society (ATS) standards [31]. Except for age and gender, other demographic characteristics were similar between the subjects who did and did not undergo spirometry. These 1,085 men and women constituted the MUDHRA cohort. Figure 2 depicts the flowchart for subject sampling and participation in different phases of the study.
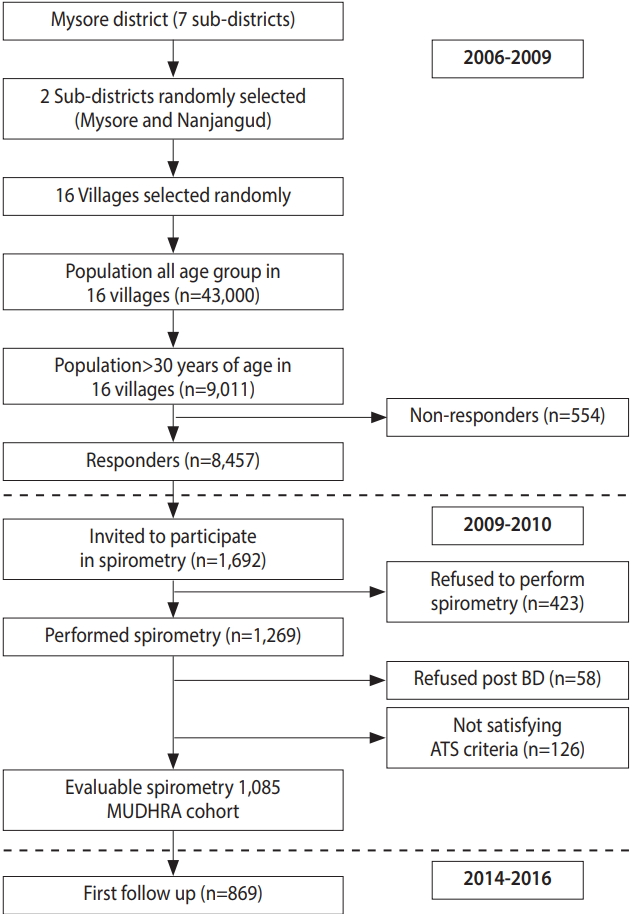
Flowchart for subject sampling and participation in different phases of the study. MUDHRA, Mysuru stUdies of Determinants of Health of Rural Adults; BD, broncho-dilator; ATS, American Thoracic Society.
After the baseline assessment for chronic lung disorders and their risk factors during 2006-2010, the cohort was retraced and examined 5 years later between 2014 and 2016. The follow-up evaluation of the cohort was conducted to establish the incidence rates of COPD, its predictors, and other chronic lung disorders. The baseline assessments were repeated and 869 subjects had acceptable spirometry results in the follow-up examination.
During this follow-up, a nested case-control design was used, and a subset of participants (n= 200; 100 with COPD and 100 without) were evaluated further to examine the hypothesis that lower levels of vitamin D and oxidative stress markers and higher levels of depressive symptoms were associated with a greater decline in lung function (manuscript under preparation).
In a smaller subset of tobacco smokers with and without COPD (n = 50), we examined the serum levels of 8 cytokines. Later, in another subset of the cohort (n= 98), we examined the levels and interactions of 40 serum cytokines and chemokines using a multiplex immunoassay system in subjects with COPD related to tobacco smoking (men) and exposure to smoke from biomass fuels (women) compared to subjects exposed to similar levels of risk factors (tobacco smoking in men and biomass smoke exposure in women) but who had not developed COPD to assess whether the immune inflammatory signatures associated with tobacco smoking-related and biomass fuel exposure-related COPD were different (manuscript submitted) [39].
MEASUREMENTS
The demographic, socioeconomic, health-related, respiratory symptom-related, risk factors, spirometry, and lab variables measured during the different phases are listed in Table 1. Chronic bronchitis was defined as having cough with phlegm on most days for 3 months for at least 2 consecutive years [2]. COPD was defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometry guidelines as a post-bronchodilator ratio of forced expiratory volume to forced lung capacity (FEV1/FVC) < 0.7 [2], and asthma was defined according to the Global Initiative for Asthma spirometry guidelines as an increase of 12.0% and 200 mL in FEV1 on a post-bronchodilator spirometry test [40].
KEY FINDINGS
Of the 8,457 men and women screened for establishing this cohort, the prevalence of cough and phlegm for a 1-month duration was 14.3%, that of chronic cough and phlegm (for 3 months’ duration) was 8.4%, and that of chronic bronchitis was 7.7%. These conditions were all more common among the elderly, men, and current smokers [41-43].
A threshold of biomass fuel exposure for an elevated risk of the diagnosis of chronic bronchitis was established for the first time in a rural population. The biomass exposure index was first described by Behera & Jindal [44], and was defined as the average hours of exposure to biomass smoke in a day multiplied by the number of years of exposure. We identified that a minimum biomass exposure index of 60 was necessary to have a significantly higher risk for developing chronic bronchitis than the general population. Increased exposure to biomass smoke increased the rates of chronic bronchitis in women [42]. Additional respiratory risk factors, such as an occupation involving contact with dust, were observed in 58.2% of subjects, and passive smoking was reported by 10.9%.
Studies have shown that biomass smoke exposure is a significant factor rivalling tobacco smoke both for the development of COPD and for mortality associated with COPD [45,46]. Increased biomass smoke exposure was associated with increasing severity of airflow limitation and advanced COPD according to the GOLD criteria [47]. The importance of biomass smoke in COPD has been further confirmed by studies that have longitudinally evaluated the effect of switching over to cleaner fuel as compared to continuing biomass smoke exposure and observed more than a 50% decrease in the risk of developing COPD [48].
Indoor levels of carbon monoxide (CO), sulphur dioxide (SO2), and nitric oxide (NO) during cooking and 3 hours thereafter were measured in 50 randomly chosen participant households. The levels of all these compounds were unacceptably high (peak CO: 999 parts per million [ppm], with a time-weighted average [TWA] of 596 ppm; peak SO2: 99.9 ppm, with a TWA of 23.4 ppm; peak NO level: 38.3 ppm, with a TWA of 5.7 ppm). At 3 hours, the levels of CO, SO2, and NO remained unacceptably high at 37, 15, and 4 ppm, respectively (manuscript under preparation).
Of the 1,085 constituent members of the cohort, 9 of the 915 men (1.0%) and 1 of the 170 women (0.6%) were diagnosed with COPD. The most common abnormality on spirometry in both gender was a restrictive defect (40.0%), and this was significantly more common among women than among men in those aged less than 40 years (28.0% of men vs. 43.0% in women). The prevalence of asthma confirmed by spirometry was 6.9% (75 of 1,085) in our study (manuscript under preparation).
At the 5-year follow-up, 72 of the 573 participants (12.6%) with chronic bronchitis and 17 of the 296 participants (5.7%) without chronic bronchitis had died, indicating a much higher all-cause 5-year mortality rate in those with chronic bronchitis (manuscript under preparation).
The nested case-control study (n=98) identified the chemokines chemokine (C-C motif) ligand (CCL)20, CCL27, and chemokine (CX-C motif) ligand (CXCL)13 as putative, plausibly homeostatic biomarkers for biomass smoke-induced COPD. Ten cytokines and chemokines exhibited higher concentrations in the tobacco smokeexposed controls than in the tobacco smoke-exposed COPD cases. A comparison of cytokine and chemokine concentrations between the biomass smoke-induced COPD subjects and the tobacco smoke-induced COPD subjects and the corresponding subjects who were exposed to biomass smoke or tobacco smoke but did not develop COPD also revealed distinct molecular profiles [49] (manuscript submitted).
STRENGTHS AND WEAKNESSES
The main strength of the MUDHRA cohort is that it is population-based and representative of the rural population in southern India. This is the single largest rural cohort in India in which all participants underwent standardized assessments for the diagnosis of COPD according to ATS criteria, with a repeat assessment for lung function at a 5-year follow-up. Very few declined to participate at baseline (< 5%) and in the follow-up studies (< 7%). We measured CO, SO2 and NO in a subset of households. Particulate matter levels (PM2.5, PM10) were not measured. Participants were not examined for cardio-metabolic disorders. Although all-cause mortality was reported for the cohort at the 5-year follow-up, the cause of death was not ascertained. We intend to overcome this by establishing the probable cause of death by conducting a standardised verbal autopsy interview with a reliable informant of the deceased and recording the cause of death from medical records or the death certificate.
DATA ACCESSIBILITY
The study data are not freely available, but the MUDHRA cohort team would welcome collaborations with other researchers. For further information, contact Dr. Mahesh PA based at JSS Medical College, JSS Academy of Higher Education and Research, Mysuru, India (mahesh1971in@yahoo.com).
Acknowledgements
We sincerely acknowledge the subjects for their kind consent and cooperation. We also thank the Principal, JSS Medical College, JSS Academy of Higher Education and Research, field staff Sathish Chandran M, Raju M (spirometry technicians), Shobha (lab technician), and Lingambika (data entry), who assisted in field and lab work and the grama panchayat members for their coordination. We also thank Dr. AK Prabhakar, Senior Epidemiologist, and Dr. MVSST Subba Rao, Associate Professor of Biochemistry from JSS Academy of Higher Education and Research, and Dr. S Ravi, Professor of Statistics from Mysore University for their valuable input.
This work was supported by the Indian Council of Medical Research (ICMR), India (grant no. 5/8/4-4[Env]/2003-NCD-I [2006-2011]); National Institutes of Health (NIH) Fogarty International Center (FIC) Global Health Equity Scholars (GHES) USA, (R25 TW009338, D43 TW010540) and the systemic biomarkers analysis by grants DBT-India (BT/PR12987/INF/22/205/2015 and VINNOVA [2016-01951] to K.G). The funding agency had no say in the conduct of the study, design of the study, analysis of the data and writing the manuscript.
Notes
The authors have no conflicts of interest to declare for this study.