Articles
- Page Path
- HOME > Epidemiol Health > Volume 36; 2014 > Article
-
LECTURE
T cell immunosenescence, hypertension, and arterial stiffness - Hee Tae Yu, Eui-Cheol Shin
-
Epidemiol Health 2014;36:e2014005.
DOI: https://doi.org/10.4178/epih/e2014005
Published online: May 23, 2014
Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, Daejeon, Korea
- Correspondence: Eui-Cheol Shin Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, 291 Daehak-ro, Yuseong-gu, Daejeon 305-701, Korea Tel: +82-42-350-4236, Fax: +82-42-350-4240, E-mail: ecshin@kaist.ac.kr
• Received: April 30, 2014 • Accepted: May 12, 2014
Copyright © 2014, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
ACKNOWLEDGEMENTS
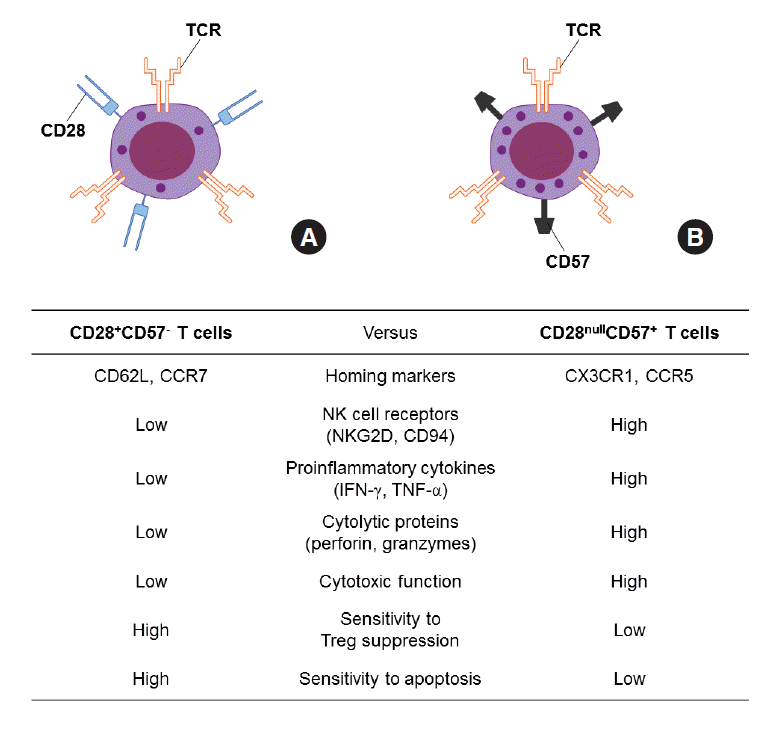
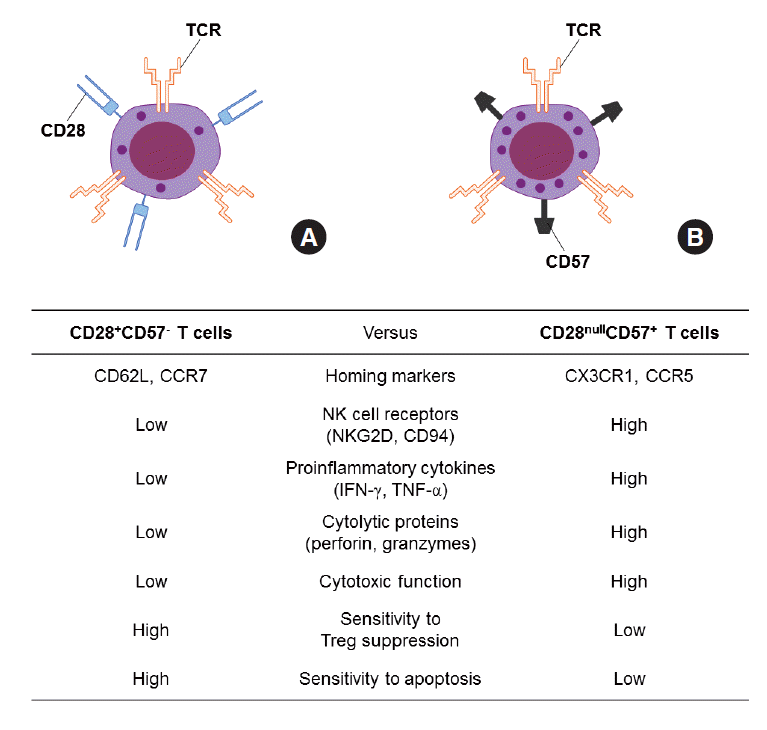
Figure 1.Comparison of CD28+CD57- T cells (A) and CD28nullCD57+ senescent T cells (B). INF, interferon; TNF, tumor necrosis factor.

- 1. Walford RL. The immunologic theory of aging. Immunol Rev 1969;2:17.Article
- 2. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol 2004;5:133-139.ArticlePubMed
- 3. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006;24:1159-1169.ArticlePubMed
- 4. Ostan R, Bucci L, Capri M, Salvioli S, Scurti M, Pini E, et al. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation 2008;15:224-240.ArticlePubMed
- 5. Lindstrom TM, Robinson WH. Rheumatoid arthritis: a role for immunosenescence? J Am Geriatr Soc 2010;58:1565-1575.ArticlePubMedPMC
- 6. Nikolich-Zugich J. T cell aging: naive but not young. J Exp Med 2005;201:837-840.ArticlePubMedPMC
- 7. Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol 2008;8:512-522.ArticlePubMedPMC
- 8. Monteiro J, Batliwalla F, Ostrer H, Gregersen PK. Shortened telomeres in clonally expanded CD28-CD8+ T cells imply a replicative history that is distinct from their CD28+CD8+ counterparts. J Immunol 1996;156:3587-3590.ArticlePubMedPDF
- 9. Effros RB. Loss of CD28 expression on T lymphocytes: a marker of replicative senescence. Dev Comp Immunol 1997;21:471-478.ArticlePubMed
- 10. Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol 2005;17:480-485.ArticlePubMed
- 11. Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol 2009;19:47-56.ArticlePubMed
- 12. Weng NP, Akbar AN, Goronzy J. CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol 2009;30:306-312.ArticlePubMedPMC
- 13. Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol 2010;87:107-116.ArticlePubMed
- 14. Vogel M, Kowalewski HJ, Zimmermann H, Janetzko A, Margolis RU, Wollny HE. Association of the HNK-1 epitope with 5’-nucleotidase from Torpedo marmorata (electric ray) electric organ. Biochem J 1991;278:199-202.ArticlePubMedPMC
- 15. Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 2003;101:2711-2720.ArticlePubMed
- 16. Dumitriu IE, Araguás ET, Baboonian C, Kaski JC. CD4+ CD28 null T cells in coronary artery disease: when helpers become killers. Cardiovasc Res 2009;81:11-19.ArticlePubMed
- 17. Maeda T, Yamada H, Nagamine R, Shuto T, Nakashima Y, Hirata G, et al. Involvement of CD4+,CD57+ T cells in the disease activity of rheumatoid arthritis. Arthritis Rheum 2002;46:379-384.ArticlePubMed
- 18. Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol 2005;175:8415-8423.ArticlePubMed
- 19. Palmer BE, Mack DG, Martin AK, Maier LA, Fontenot AP. CD57 expression correlates with alveolitis severity in subjects with beryllium-induced disease. J Allergy Clin Immunol 2007;120:184-191.ArticlePubMed
- 20. Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet 2001;358:472-473.ArticlePubMed
- 21. Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 2003;23:842-846.ArticlePubMed
- 22. Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis 2011;203:452-463.ArticlePubMedPMC
- 23. Liuzzo G, Kopecky SL, Frye RL, O’Fallon WM, Maseri A, Goronzy JJ, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation 1999;100:2135-2139.ArticlePubMed
- 24. Liuzzo G, Biasucci LM, Trotta G, Brugaletta S, Pinnelli M, Digianuario G, et al. Unusual CD4+CD28null T lymphocytes and recurrence of acute coronary events. J Am Coll Cardiol 2007;50:1450-1458.ArticlePubMed
- 25. Giubilato S, Liuzzo G, Brugaletta S, Pitocco D, Graziani F, Smaldone C, et al. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J 2011;32:1214-1226.ArticlePubMed
- 26. Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension 2013;62:126-133.ArticlePubMed
- 27. Kannel WB, Wolf PA, McGee DL, Dawber TR, McNamara P, Castelli WP. Systolic blood pressure, arterial rigidity, and risk of stroke. The Framingham study. JAMA 1981;245:1225-1229.ArticlePubMed
- 28. Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension 1998;32:570-574.ArticlePubMed
- 29. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 2005;46:1118-1122.ArticlePubMed
- 30. Tuttolomondo A, Di Raimondo D, Pecoraro R, Serio A, D'Aguanno G, Pinto A, et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis 2010;213:311-318.ArticlePubMed
- 31. Wall NA, Chue CD, Edwards NC, Pankhurst T, Harper L, Steeds RP, et al. Cytomegalovirus seropositivity is associated with increased arterial stiffness in patients with chronic kidney disease. PLoS One 2013;8:e55686.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations
Citations to this article as recorded by 

- Pre-Existing Hypertension Is Related with Disproportions in T-Lymphocytes in Older Age
Anna Tylutka, Barbara Morawin, Artur Gramacki, Agnieszka Zembron-Lacny
Journal of Clinical Medicine.2022; 11(2): 291. CrossRef - Blood pressure variability: A potential marker of aging
Leonardo Bencivenga, Philipe De Souto Barreto, Yves Rolland, Olivier Hanon, Jean-Sébastien Vidal, Philippe Cestac, Bruno Vellas, Laure Rouch
Ageing Research Reviews.2022; 80: 101677. CrossRef - Cluster Analysis of Inhalant Allergens in South Korea: A Computational Model of Allergic Sensitization
Dong-Kyu Kim, Young-Sun Park, Kyung-Joon Cha, Daeil Jang, Seungho Ryu, Kyung Rae Kim, Sang-Heon Kim, Ho Joo Yoon, Seok Hyun Cho
Clinical and Experimental Otorhinolaryngology.2021; 14(1): 93. CrossRef - Research progress on immune aging and its mechanisms affecting geriatric diseases
Yanping Yu, Songbai Zheng
AGING MEDICINE.2019; 2(4): 216. CrossRef - Cytomegalovirus driven immunosenescence—An immune phenotype with or without clinical impact?
Cecilia Söderberg-Nauclér, Olesja Fornara, Afsar Rahbar
Mechanisms of Ageing and Development.2016; 158: 3. CrossRef

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite