Articles
- Page Path
- HOME > Epidemiol Health > Volume 44; 2022 > Article
-
Systematic Review
Systematic review and meta-analysis of cancer risks in relation to environmental waste incinerator emissions: a meta-analysis of case-control and cohort studies -
Kiook Baek1
 , Jong-Tae Park2
, Jong-Tae Park2 , Kyeongmin Kwak2
, Kyeongmin Kwak2
-
Epidemiol Health 2022;44:e2022070.
DOI: https://doi.org/10.4178/epih.e2022070
Published online: September 1, 2022
1Department of Occupational and Environmental Medicine, Yeungnam University Hospital, Daegu, Korea
2Department of Occupational and Environmental Medicine, Korea University Ansan Hospital, Ansan, Korea
- Correspondence: Kyeongmin Kwak Department of Occupational and Environmental Medicine, Korea University Ansan Hospital, 123 Jeokgeum-ro, Danwon-gu, Ansan 15355, Korea E-mail: pathfinder81@korea.ac.kr
©2022, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,904 Views
- 219 Download
- 2 Web of Science
Abstract
-
OBJECTIVES
- Various toxic substances can be generated from incinerators, exposing nearby residents, and epidemiological studies have shown wide variations in risk estimates for cancer risk in populations living close to incinerators.
-
METHODS
- Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, a literature search and systematic review were conducted to identify studies conducted on general populations exposed to environmental incinerator emissions and cancer outcomes. Meta-analysis was performed according to the cancer types for which 2 or more studies were reported. Subgroup analysis was done for sex, the exposure estimation method, the study period, and the type of outcome.
-
RESULTS
- Eleven studies were found for the qualitative review and meta-analysis. Seven studies had a case-control design, and 4 had a cohort design. The pooled effect size was not significant for breast, colorectal, liver, lung, lymphohematopoietic, stomach, bladder, central nervous system, and laryngeal cancers, non-Hodgkin lymphoma, sarcoma, leukemia, and all cancers. In the subgroup analysis, the pooled effect size of laryngeal cancer in females was 1.82 (95% confidence interval, 1.10 to 3.01), although only 2 studies were identified.
-
CONCLUSIONS
- The meta-analysis did not provide evidence of an increased risk for any cancer among populations living near waste incinerators, except for laryngeal cancer in females. However, since relatively few studies were reviewed and some cancer types showed significant increases in individual studies, this evidence needs to be updated regularly.
- Populations living near incinerators may be exposed to various pollutants (e.g., ash, odor, dust, or spores) [1], including carcinogens such as dioxins and heavy metals [2,3]. Although regulations on incinerators have been strengthened and pollutant emissions and exposures are decreasing [4,5], concerns regarding the potential health effects of living near incinerators persist. To date, no clear consensus exists on the increase in cancer risk owing to long-term exposure to low concentrations of carcinogens [6]. Debate continues regarding the extrapolation and application of the estimated carcinogenic effect at high concentrations to the risk of cancer in settings of low-dose exposure [7]. It is challenging to confirm the level or even the existence of the carcinogenic threshold at low concentrations of toxicants, and the estimation of carcinogenic risk through extrapolation is also uncertain [8].
- Many studies, including well-designed ones, have reported that living near incinerators poses a cancer risk; however, some studies with relatively unsophisticated designs have also shown a low evidence level [9]. It is impossible and inefficient for the public and decision-makers to review all the literature and data accumulated to date. In addition, there is a risk of selectively obtaining and citing research results that support different claims. Thus, studies with a high level of evidence and integrated results are warranted.
- Despite a large number of systematic reviews on the risk of environmental exposure to toxicants from incinerators [10-12], no meta-analysis has been reported. Therefore, we performed a systematic review and meta-analysis of case-control and cohort studies among populations living near incinerators, focusing on cancer risk.
INTRODUCTION
- Study design, protocol, and registration
- This systematic review and meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [13,14]. The protocol was registered in the International Prospective Register of Systematic Reviews on January 5, 2022 (CRD42022292049) [15].
- Literature search
- PubMed, Embase, and Web of Science were searched for articles published up to December 3, 2021. The search strategy for each database used during the literature search is shown in Table 1. Those with duplicate data were excluded; the title and abstract and then the full-text were checked in accordance with the inclusion and exclusion criteria described below. References from the included literature and the literature cited in the included literature were manually checked to retrieve reports from the initial search using the same method that was used for assessing the initial search results. Individual studies and the corresponding extracted data were reviewed independently by 2 unblinded authors, KB and KK. If there were different opinions on the inclusion or exclusion of the papers, a vote was taken by all 3 authors.
- Inclusion criteria
- We included studies on the increase in human cancer incidence among general populations living near incinerators or those exposed to emissions from incinerators, which provided risk estimates and confidence intervals (CIs) for comparisons between non-dose or low-dose exposure groups. We included studies reporting any human cancer risk outcome. Studies with all types of incinerators were searched, regardless of the construction period, generation, or incineration material. Only case-control and cohort studies were included.
- Exclusion criteria
- The following studies were excluded: ecological studies; animal studies; studies on occupational exposure to incinerator emissions; studies on risk assessments or exposure assessments without cancer-related data; review articles (including systematic reviews); non-original research (such as case reports, case series, commentaries, and conference abstracts); and studies on general industrial pollution wherein incinerator emissions were not evaluated.
- Data extraction
- From the included studies, data regarding the name of the first author, publication year, sex of the participants, study design, region, size, period of the study, outcomes, exposure assessment method, effect size, and 95% CIs were extracted. If an exposure was classified into several exposure levels, the effect size of the lowest compared with that of the highest estimated exposure group was extracted. When the exposure was classified based on the distance from the incinerator, the effect size of the nearest area compared with that of the farthest from the incinerator was extracted. If the effect size and CI were not provided explicitly in a study, they were calculated using data presented in the article. Cases in which several effect sizes were stratified by sex or age were pooled. Among studies reporting both adjusted and unadjusted values for confounders, the adjusted values were extracted.
- Quality assessment
- We evaluated each article using the Newcastle–Ottawa Scale (NOS). The NOS contains 8-item categories in three components: selection, comparability, and outcome (for cohort studies) or exposure (for case-control studies) [16]. It is scored on a 0-point to 9-point scale, with 7-9 points indicating high quality, 4-6 points indicating intermediate quality, and 0-3 points indicating low quality [17]. Individual studies were independently assessed by 2 authors: KK and KB. If there were different opinions on whether to include or exclude a study, a vote was taken by all authors.
- Statistical analysis
- The pooled effect size and the corresponding 95% CIs were calculated for each cancer type reported in at least 2 studies. The effect sizes for all cancer types were pooled. Although the outcomes of the studies that included various cancers were heterogeneous, this method has been previously used in the literature [18,19]. If duplicate effect sizes were reported, the effect size for the upper category was extracted and analyzed. Although the effect sizes varied in terms of whether they were reported as the hazard ratio (HR), rate ratio (RR), or odds ratio (OR), the results were pooled together in consideration of the rare disease assumption [20]. RRs and HRs for incidence or hospitalization from cohort studies were pooled with ORs from case-control studies. A random-effects model was used considering the heterogeneity of environmental epidemiological studies [21]. The I2 statistics and p-values of the Cochran Q test for each analysis are presented. Funnel plots and the Egger test were used to evaluate publication bias. Meta-analyses were conducted using Stata version 15 (StataCorp., College Station, TX, USA) with the “metan” command. Publication bias was assessed using R version 3.6.3 (https://R-project.org) with the “metafor” package.
- Subgroup analysis
- The following subgroup analyses were performed: (1) A meta-analysis was done with stratification by sex for studies that reported the effect size by sex. (2) Since the method of exposure estimation was different for each study, cases where the exposure was evaluated by modeling emissions components were analyzed separately. (3) A meta-analysis was performed of studies reporting the effect size for mortality. (4) A meta-analysis was performed according to the time of the start of exposure (before and after 2000), considering the lower pollution emissions of recently built incinerators [11].
- Ethics statement
- No ethical approval is required since this study was based on published articles and did not involve human subjects.
MATERIALS AND METHODS
- Characteristics of the included studies
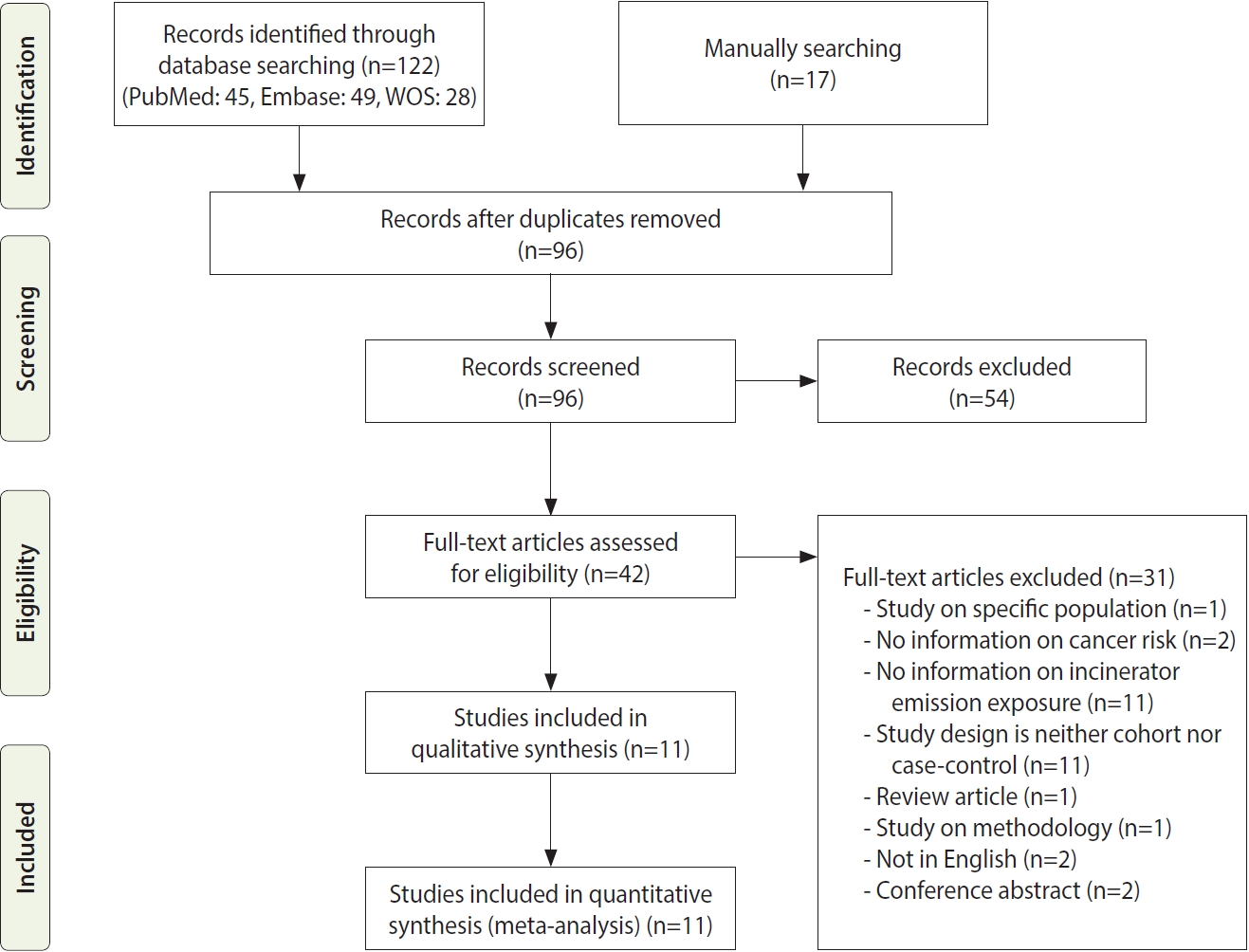
- With search terms, 122 studies were identified from databases. Sixteen studies were manually searched from citations and references. Duplicate publications were removed, and the remaining articles were screened. We excluded 54 articles after screening their abstracts and reviewed the full-text of 42 articles. Articles for which we performed full-text review are presented in Supplementary Material 1. Finally, 11 articles were included in the final literature review and meta-analysis (Figure 1).
- Briefly, 7 were case-control studies, and 4 were cohort studies. The study periods spanned from 1979 to 2015. Among the cohort studies, 1 study reported only the incidence; 2 studies reported mortality and hospitalization; and 1 study reported mortality, incidence, and hospitalization as outcomes. ORs were extracted from the case-control studies. As for the types of incineration facilities, exposure evaluation was performed for municipal waste in 6 studies, industrial waste in 2, and medical, municipal, sewage, and hazardous waste in 1. There was no mention of the type of incinerator in 1 study. One study evaluated incinerators and industrial exposure without distinction [22]; however, the incinerator accounted for a large proportion of exposure, and the exposure assessment (performed using dispersion modeling) was of high quality; thus, we included this study. One case-control study reported the modeling of the-estimated relative risk according to the distance from the incinerator in cases based on lung cancer autopsy results [4]. Other detailed information on each study is presented in Table 2. The NOS evaluation results are provided as online Supplementary Material 2.
- Effect size extraction and meta-analysis
- ORs from case-control studies and RRs/HRs from cohort studies were extracted and pooled. Pronk et al. [23] reported the effect sizes of 4 incinerators (medical, municipal, sewage, and hazardous waste) and other industrial facilities. Because the exposed populations of the 4 incinerators overlapped, the effect size (OR) for medical waste incinerators, which caused the most exposure, was extracted.
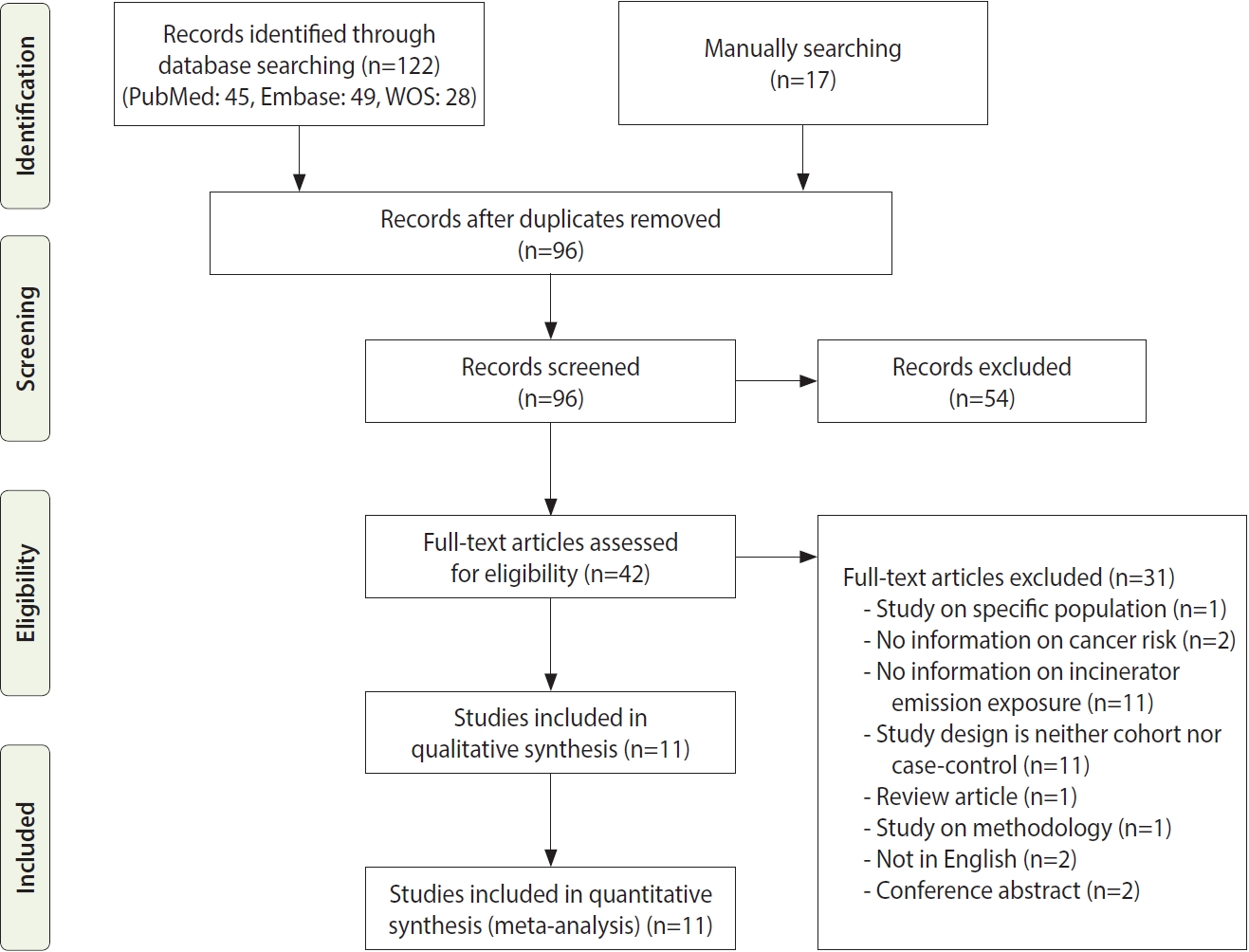
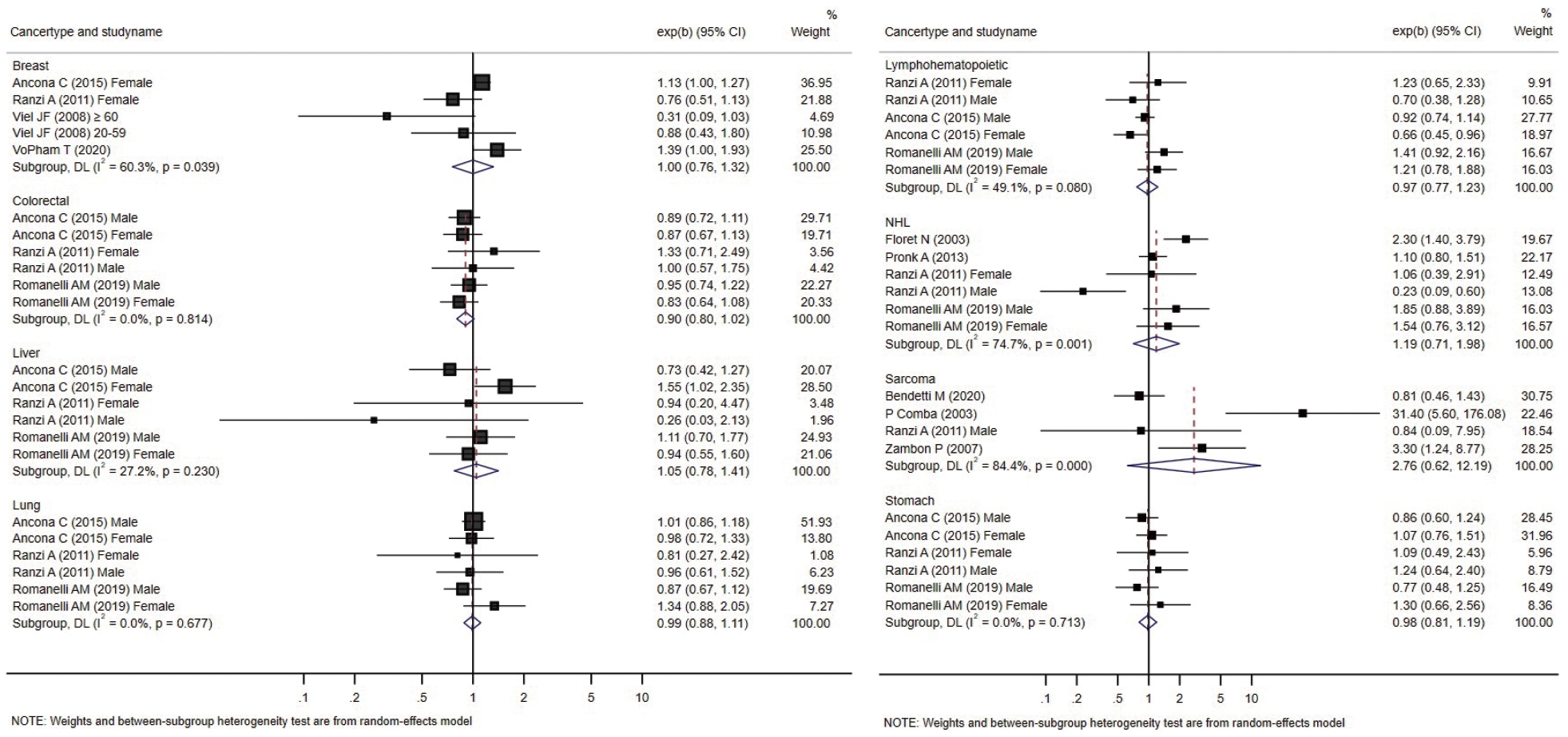
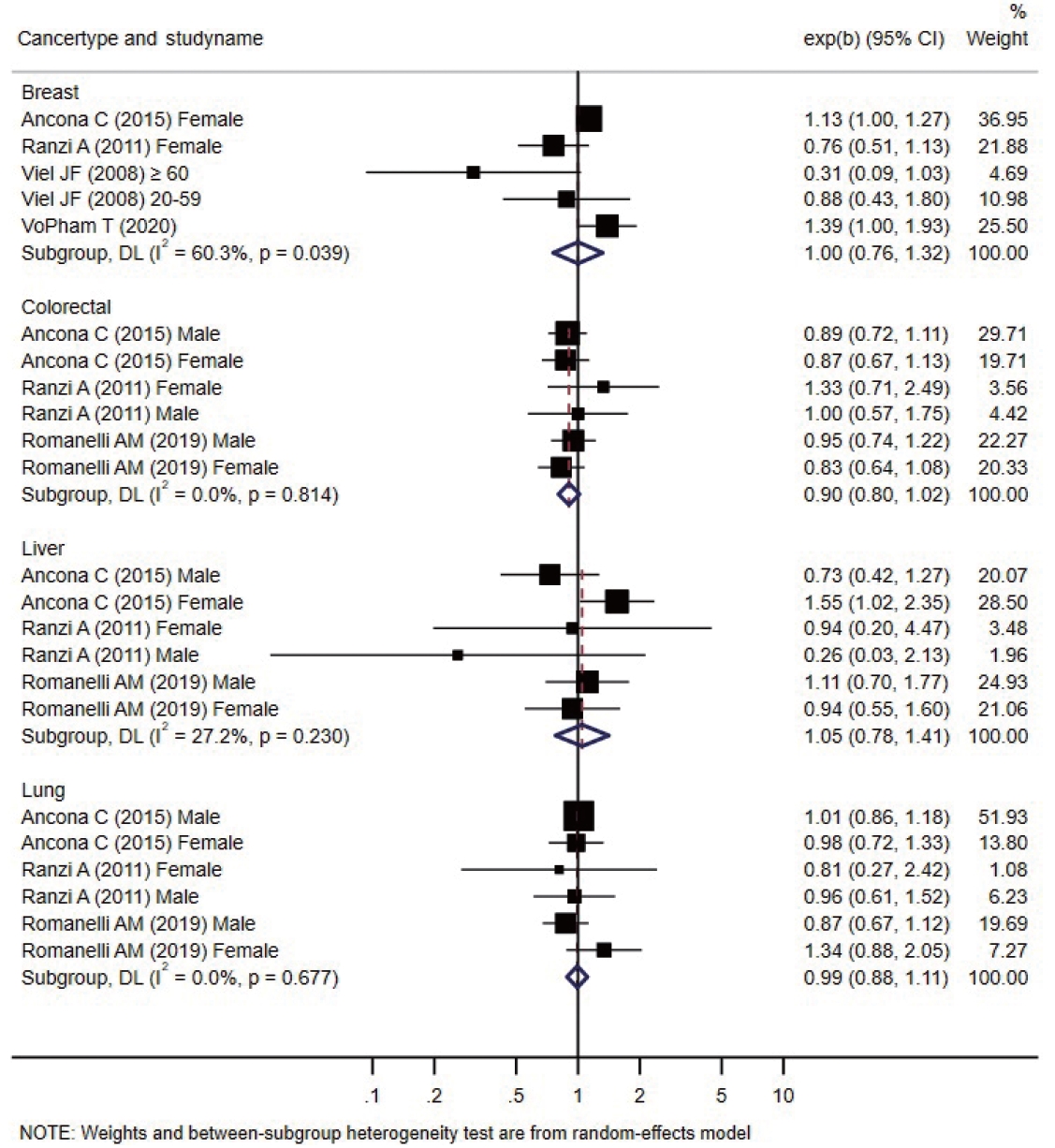
- Forest plots presenting individual and pooled effect sizes for breast, colorectal, liver, lung, lymphohematopoietic, and stomach cancers, as well as non-Hodgkin lymphoma (NHL) and soft tissue sarcoma, are presented in Figure 2. Forest plots for the outcomes reported in 2 studies (bladder, central nervous system [CNS], laryngeal cancers, leukemia, and all cancers) are presented in Figure 3. The meta-analytic pooled effect sizes for all cancer types combined are shown in Supplementary Material 3. A funnel plot and coefficient from the Egger test are presented in Supplementary Material 4.
- Summary by cancer type
- The risk of breast cancer was reported in 4 studies, all performed in females. VoPham et al. [24] reported that the incidence of breast cancer was significantly higher in individuals living near incinerators among a prospective cohort of nurses. For individuals living within 3 km, 5 km, and 10 km of municipal solid waste incinerators, the HRs were 1.20 (95% CI, 0.86 to 1.68), 1.25 (95% CI, 1.04 to 1.52), and 1.15 (95% CI, 1.03 to 1.28), respectively. The HRs tended to increase as the period of residence near the incinerator increased. Compared with the group without a period of residence at a distance of 3 km near municipal solid waste incinerators, the HR increased by 1.07 (95% CI, 0.77 to 1.49) for 1-6 years and 1.39 (95% CI, 1.00 to 1.93) for those with more than 6 years of residence. In a case-control study conducted by Viel et al. [25], the results were stratified between the 20-year to 59-year and ≥ 60-year age groups, and no significant relationship with residence near incinerators was found in either group. Two retrospective cohort studies did not confirm a significant relationship [26,27]. The pooled effect size was not significant.
- In a case-control study conducted in France by Floret et al. [28], there was a significantly higher incidence of NHL in individuals living near incinerators. In another case-control study from the United States no significant difference was identified in a case-control study reported later [23]. Two retrospective cohort studies reported risk estimates for NHL. Romanelli et al. [29] reported no significant increase in the HRs, while Ranzi et al. [27] reported that the risk of NHL was significantly lower in males living near incinerators. The pooled effect size was not significant.
- In a case-control study conducted by Comba et al. [30], the OR for soft tissue sarcoma was calculated according to the distance from an incinerator, and the result obtained was significant (OR, 31.4; 95% CI, 5.6 to 176.1) for the group living within 2 km of an incinerator compared to the group living more than 5 km away. There were 5 cases and 1 control in the group residing within 2 km of an incinerator and 7 cases and 44 controls in the group residing more than 5 km away.
- In 2007, Zambon et al. [22] conducted a case-control study in Italy and calculated the OR considering the estimated dioxin exposure level and period through dispersion modeling. The study included 172 patients and 405 controls. The OR (3.30; 95% CI, 1.24 to 8.77) was significantly higher in the group exposed to ≥ 6 fg/m3 of polychlorinated dibenzo-p-dioxins and dibenzofurans for more than 32 years than in the group exposed to < 4 fg/m3 of polychlorinated dibenzo-p-dioxins and dibenzofurans for less than 32 years. Ten cases and 44 controls were exposed to < 4 fg/m3 of dioxin for less than 32 years, while 20 cases and 26 controls in the control group were exposed to ≥ 6 fg/m3 of dioxin for ≥ 6 years. In the assessment that did not consider the exposure period, a significant result (OR, 2.41; 95% CI, 1.04 to 5.59) was reported in the female group with exposure to ≥ 6 fg/m3 of dioxin compared to that in the group with exposure to < 4 fg/m3 of dioxin. However, in the study by Benedetti et al. [31], residential history was classified in various manners, but no significant increase was observed with any method. The OR calculated in the exposure group for the population exposed after 1961, excluding 10 years before the diagnosis, was 0.81 (95% CI, 0.46 to 1.43); when the time-window and high exposure period (1961-1991) ere considered in the exposure history, the analysis showed no significant decrease in risk (OR, 0.57; 95% CI, 0.27 to 1.21). The pooled effect size was not significant.
- Colorectal, stomach, lymphohematopoietic, and liver cancers were reported in 3 retrospective cohort studies [26,27,29] that analyzed various cancers; no significant increase in the incidence or mortality was observed in individual studies. All 3 studies were conducted in Italy. Ancona et al. [26] reported the outcomes in Rome based on data from the regional hospital information system and the regional registry of causes of death. Ranzi et al. [27] used data from the cancer database, hospital admissions database, and regional mortality database in Forlì. Romanelli et al. [29] reported cancer outcomes based on the regional hospital information system and regional mortality registry data in Pisa. In all 3 studies, codes from the International Classification of Diseases (ICD), ninth revision, were used to analyze the cancer type as a health outcome. The pooled effect sizes were not significant.
- Three retrospective cohort studies [26,27,29] reported non-significant changes in lung cancer-specific incidence or mortality. The pooled effect size was not significant. There was no significant increase in the pooled effect size in the meta-analysis.
- Biggeri et al. [4] conducted a case-control study that analyzed the change in relative risk according to the distance from the city center, an iron foundry, a shipyard, and an incinerator, with modeling conducted through a point source analysis. Even after adjustments for individual risk factors, the maximum OR (5.9, p< 0.001) was estimated at the point of the incinerator and decreased sharply with distance. However, because this value was not the actual outcome of research and the case definition was death (autopsy), we excluded this study from the meta-analysis presented in Figure 2. This study was pooled in the subgroup meta-analysis for mortality.
- Two studies reported the effect size of living near incinerators on laryngeal cancer incidence. A significant increase in laryngeal cancer-specific mortality and hospital admissions was reported among females in a retrospective cohort study conducted by Ancona et al. [26]. Significant differences in mortality (OR, 1.92; 95% CI, 1.16 to 3.19) and hospital admissions (OR, 1.83, 95% CI, 1.09 to 3.06) were reported among the females exposed to waste incinerator emissions in the cohort. Exposure estimation was modeled using particulate matter less than 10 μm (PM10). The OR was calculated as the difference between the 95th and 5th percentiles. No significant elevation in mortality or hospital admissions was observed among males. Ranzi et al. [27] reported a non-significant association between laryngeal cancer risk and living close to incinerators. Exposure estimation was performed using dispersion modeling for heavy metal exposure. RRs were calculated by stratifying the estimated exposure by quartiles. The number of cases identified as laryngeal cancer was 18 in the first quartile of exposure and 1 in the fourth quartile of exposure. Among males, the RR was 0.15 (95% CI, 0.02 to 1.13). The incidence of laryngeal cancer was 2 in the first quartile of exposure and 1 in the fourth quartile of exposure; and the RR was 1.60 (95% CI, 0.15 to 17.35). In the same study, the RR for mortality could not be calculated because there were no deaths in the first and fourth quartiles among females, while 0 deaths and 6 deaths were observed in the fourth and first quartiles among males, respectively.
- Two retrospective cohort studies [26,27] analyzed the risk of bladder cancer in relation to living near incinerators and found no significant results. The results were shown for males and females separately.
- CNS cancer was analyzed in 2 retrospective cohort studies [27,29]. In a study with dispersion modeling for heavy metal exposure [27], the risk excess was not significant (RR, 1.35; 95% CI, 0.34 to 5.39) among males comparing the groups with the lowest and highest exposures. No cases were identified in the highest exposure group among females. Romanelli et al. [29] reported a non-significant increase in the incidence of CNS cancer in males (HR, 1.87; 95% CI, 0.54 to 6.44) and a non-significant reduction in CNS cancer incidence in females (HR, 0.38; 95% CI, 014 to 1.05).
- Some studies reported the risk of leukemia and myeloma separately from that of lymphohematopoietic cancer. Leukemia was reported in 2 studies [27,29], and the risk was presented separately for males and females; however, no significant difference was found in effect size.
- Studies reporting the risk for all cancers in the original text were analyzed. Two studies calculated the effect size by integrating all types of cancers. Two retrospective cohort studies [26,27] reported the effect size of all cancers based on ICD codes among cohort participants. One study used ICD codes 140-239 to define all cancers [27], while another study used ICD codes 140-208 [26]. The effect size was presented separately by sex. In the meta-analysis, the 95% CI for the pooled effect size overlapped with the null hypothesis.
- Although the heterogeneity of the outcome was severe, in the meta-analysis process, the effect size of incidence was pooled regardless of the type of cancer. The pooled effect size was 1.00 (95% CI, 0.94 to 1.06), which was not statistically significant.
- Subgroup analysis
- The results of the subgroup analysis are presented in Supplementary Material 5. In the meta-analysis that analyzed only the mortality outcome, a significant increase or decrease in the effect size was not observed. No significant increase or decrease in the effect size was observed in the meta-analysis of studies that estimated exposure through modeling. When stratified based on the study period (time of enrollment), the effect size for cancer did not show a statistically significant result.
- In the subgroup analysis by sex, the risk of laryngeal cancer was significantly increased in females (effect size, 1.82; 95% CI, 1.10 to 3.01) with pooled results from 2 studies. Except for laryngeal cancer in females, there were no statistically significant results in the analysis stratified by sex.
RESULTS
Breast cancer
Non-Hodgkin lymphoma
Soft tissue sarcoma
Colorectal, stomach, lymphohematopoietic, and liver cancers
Lung cancer
Laryngeal cancer, bladder cancer, central nervous system cancer, and leukemia
All cancers
- The articles reviewed in this study were case-control and cohort studies, which are regarded to have relatively high epidemiological evidence levels among observational study designs. However, the results were inconsistent and varied, which could cause confusion among the public and experts; thus, the need for quantitative pooling of studies has been raised. We conducted a systematic review and meta-analysis of 11 studies presenting the risk of cancer in general populations living near incinerators. There have been reports of clusters of various cancer types related to residence near a waste management site [32], and research continues to be conducted on the increase in cancer incidence in populations residing near incinerators. Individual studies have reported increases in the risk of sarcoma [30], NHL [28], lung cancer [4], laryngeal cancer, and pancreatic cancer [26]. No significant results were noted in a meta-analysis of cancers identified in 2 or more studies. In the subgroup analysis, the pooled risk of laryngeal cancer in females reported in two studies showed statistically significant results, though only 2 studies presented data on laryngeal cancer risk in females. Associations of laryngeal cancer with dioxins [33], PM10 [34], and heavy metals [35] have been reported. Although epidemiologically, laryngeal cancer is a rare disease, it has been suggested to be related to residence near an incinerator since 1990; however, the evidence for this has not been sufficient [36]. There have also been inconsistencies in the evidence gathered since then. Several studies that were not included in this systematic review process due to their ecological study design have reported changes in the risk of laryngeal cancer with residence near an incinerator. One study showed that distance from a plant with an incinerator was associated with the standardized mortality ratio for laryngeal cancer, with an increasing pattern as the distance decreased from the plant area (p= 0.03). However, this result became insignificant after adjusting for socioeconomic status (p= 0.06) [37]. Other ecological-design studies reported no significant change [38] or decrease [39] in the association between the risk of laryngeal cancer and living near an incinerator. The quality of evidence and the direction of effects reported in various studies vary; thus, there is a risk of selective selection in research and the media. Although our study did not draw a clear conclusion, it will help in decision-making and set directions for future research by presenting results with a relatively high evidence level and suggesting a pooled effect. The effect of exposure caused by incinerator emissions on laryngeal cancer requires further investigation, and the accumulation of evidence should be continuously monitored.
- The substances emitted by incinerators include PM10, dioxins, heavy metals, and nitric oxide (NO) and nitrogen dioxide (NO2). Although there are some critical views on the carcinogenic effects of dioxin-like compounds [7,8], it is widely accepted that they pose a cancer risk, according to studies on high-dose exposure groups, such as workers or residents exposed to the Seveso accident [40-42]. The International Agency for Research on Cancer reported that there was sufficient evidence for the carcinogenic effect of 2, 3, 7, 8-tetrachlorodibenzo-para-dioxin for all cancers combined and limited evidence with respect to lung cancer, soft tissue sarcoma, and NHL among individual cancers in humans [43,44]. Studies have sporadically reported the association of dioxin exposure with other cancers, such as breast and rectal cancers [45,46]. However, it is difficult to confirm the dose-response curve and threshold for extrapolation to low-dose exposure [47]. Similarly, heavy metals, such as cadmium, arsenic, and chromium, are widely known carcinogens for lung cancer that can be generated in incinerators [48]. Nonetheless, recent studies on environmental exposure to flue gas near incinerators have shown that levels of pollutants are not substantially higher near incinerators than in the general atmosphere [49]. Even if exposure is higher than in a non-exposed area, it is difficult to assess the effect of long-term exposure to low concentrations of carcinogens in the environment. Although some studies have estimated the risk through substance exposure assessment [50], estimating the risk without epidemiological evidence is insufficient, and these uncertainties cause various obstacles to risk perception and risk communication in residences and establishments around incinerators [17,51,52].
- In epidemiological studies of environmental factors, exposure assessment for individuals is a challenging process [53] and has been reported heterogeneously in various studies [54]. For instance, Goria et al. [55] recommended using dispersion modeling to estimate exposure rather than the distance from the source because the results may depend on the exposure assessment methodology used in the analysis of the same data. In general, in spatial analysis, carcinogenic contaminants other than those produced by incinerators may coexist, and attempts have been made to use dispersion modeling to overcome this issue [26,29]. Various substances are generated from incinerators, and exposure estimates for carcinogens, such as dioxins, vary depending on incinerator usage and weather conditions; thus, risk assessment is difficult owing to the high level of uncertainty [10]. In the studies included in this review, distance from the source, region, and dispersion modeling were used to assess individual exposure. However, the indicator materials used for dispersion modeling were diverse, such as dioxins, heavy metals, PM10, and NOx, and the modeling methods were heterogeneous. No single tool has been optimized for assessing carcinogens or other toxicants around incinerators. The subgroup analysis was performed by pooling only cases where exposure was estimated by modeling, regardless of the modeling method; however, there was no change in the trend of the results. A more detailed subgroup analysis according to the exposure assessment method is needed, but it was impossible to proceed further because the number of studies was insufficient to stratify the method of exposure assessment.
- It is difficult to conclude that incinerator emission exposure is not associated with an increase in other cancer risks based on the results of this study alone. In our review, studies from Italy (n= 7), France (n= 2), and the United States (n= 2) were included. The European Union began to regulate incinerator emissions in earnest from the early 1990s [56], and in the United States, the regulation of incinerators was strengthened after 1995 [57]. Moreover, the degree of regulation and incineration materials are different for each country, resulting in a trend of differences in emissions. The studies included in this meta-analysis were conducted in countries with relatively strong regulations; therefore, the exposure levels were expected to be relatively low [58]. Studies with large weights, such as in our meta-analysis, are relatively recent, and timed after strict emission regulations were implemented in Europe and the United States. It is possible that the carcinogenic effect size may have been underestimated due to the relatively low exposure to emissions in the group classified as “high exposure.” It is necessary to update research results for regions with a relatively high exposure level of pollutants caused by emissions, such as underdeveloped or developing countries. In addition, most studies classified exposure groups based on the place of residence, and even if precise modeling is performed, individual exposure cannot be perfectly estimated. Even given these technical difficulties, there are still insufficient studies that have directly measured exposures or estimated exposures using biological exposure markers.
- The limitations of this meta-analysis are that there were variations in the design (participants, exposure estimation, comparator, and outcome) among the studies, and the number of included articles was relatively small. Studies involving different periods, countries, types of incinerators, and incinerated materials were pooled. There are differences in the definitions of disease outcomes in the literature; for instance, the prevalence of sarcoma was low and there were a variety of types. In 1 study, lymphatic vessel sarcoma, nerve sheath sarcoma, and alveolar sarcoma were included in the extraction process using the ICD for Oncology-II morphology code [22], whereas in another study, it was not included [30]. Two studies reported “all cancer” risk, using different selection criteria [26,27]. In addition, there were differences in the confirmation of cancer outcomes across studies, as exemplified by the use of medical reviews, cancer registries, questionnaires, or biopsies and autopsies. Nevertheless, it was difficult to perform meta-regression or further stratified subgroup analyses for various factors because few studies have reported the effect size for each cancer. Although a subgroup analysis was performed based on sex, important general characteristics, such as age, country, and race/ethnicity, were not stratified. Another limitation is that a stratified analysis was not performed on the generation of incinerators, although it was attempted to supplement this gap by stratification according to the enrollment dates of the study subjects.
- Moreover, there was a risk of bias in each study’s use of spatial data as exposure variables. Due to the nature of spatial analyses linked to addresses, there was a risk of selection and ecological bias in reflecting actual individual exposure [21,59]. Several studies have been conducted on dioxin biomonitoring near incinerators; however, it was difficult to find case-control or cohort studies reporting both individual biomonitoring and cancer risk.
- Atmospheric exposure to toxicants is low compared to workplace standards; however, continuing exposure to air pollutants, such as PM10 and dioxin, can cause various subclinical health effects [60,61]; thus, concerns regarding the cancer risk of individuals residing near incinerators persist [62]. Authorities such as the US National Research Council [63] insist that the actual health risk of individuals residing near modern incinerators is minimal to moderate in normal, controlled operating conditions; nonetheless, uncertainty and potential risk remain. To overcome these uncertainties, continuing epidemiological and mechanistic studies, as well as systematic literature reviews of such studies, are required to provide more clear information to the public and policy-makers. It is encouraging that studies with a high evidence level that synthesize various disease data, such as health insurance data and cancer registration data, and atmospheric modeling data are being conducted, and evidence should continue to be collected and updated. The systematic search strategy used herein constitutes a resource for regular literature searchers using the suggested strategy to keep results up to date.
DISCUSSION
- Several systematic reviews on the health risks, including cancer risks, and health effects of living near incinerators have been previously conducted; however, a quantitative synthesis has not yet been performed. So far, there is a lack of evidence of elevated risk of specific cancers after pooling the effect sizes by cancer type, except for laryngeal cancer in females. However, the evidence for each cancer type is relatively small-scale; therefore, it is difficult to conclude that sufficient evidence has been gathered. It is necessary to monitor and update the evidence on a regular basis in the future.
CONCLUSION
SUPPLEMENTARY MATERIALS
Supplementary Material 2.
Supplementary Material 4.
-
DATA AVAILABILITY
The datasets analyzed during this study are included in this published article as Supplementary Material 6.
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
-
FUNDING
This study was supported by a grant from Korea University in 2022.
-
AUTHOR CONTRIBUTIONS
Conceptualization: Baek K. Data curation: Baek, K, Kwak K. Formal analysis: Baek K. Funding acquisition: Kwak K. Methodology: Baek K, Park JT. Project administration: Park JT. Visualization: Baek K. Writing – original draft: Baek K. Writing – review & editing: Kwak K, Park JT.
NOTES
ACKNOWLEDGEMENTS
NOS, Newcastle-Ottawa Scale; MWI, municipal waste incinerator; OR, odds ratio; NHL, non-Hodgkin lymphoma; PCDD, polychlorinated dibenzo-p-dioxin; PCDF, polychlorinated dibenzofuran; SO2, sulfur dioxide; RR, risk ratio; HR, hazard ratio; CNS, central nervous system; BBD, benign breast disease; BMI, body mass index; PM10, particulate matter less than 10 μm; NO2, nitrogen dioxide; SEP, socioeconomic position; NOx, nitric oxide (NO) and nitrogen dioxide (NO2); IND, industrial plant; TR, traffic-related.
- 1. Rushton L. Health hazards and waste management. Br Med Bull 2003;68:183-197.ArticlePubMed
- 2. Valberg PA, Drivas PJ, McCarthy S, Watson AY. Evaluating the health impacts of incinerator emissions. J Hazard Mater 1996;47:205-227.Article
- 3. Wheatley A, Sadhra S. Carcinogenic risk assessment for emissions from clinical waste incineration and road traffic. Int J Environ Health Res 2010;20:313-327.ArticlePubMed
- 4. Biggeri A, Barbone F, Lagazio C, Bovenzi M, Stanta G. Air pollution and lung cancer in Trieste, Italy: spatial analysis of risk as a function of distance from sources. Environ Health Perspect 1996;104:750-754.ArticlePubMedPMC
- 5. Liu A, Ren F, Lin WY, Wang JY. A review of municipal solid waste environmental standards with a focus on incinerator residues. Int J Sustain Built Environ 2015;4(2):165-188.Article
- 6. Bevan RJ, Harrison PT. Threshold and non-threshold chemical carcinogens: a survey of the present regulatory landscape. Regul Toxicol Pharmacol 2017;88:291-302.ArticlePubMed
- 7. Cole P, Trichopoulos D, Pastides H, Starr T, Mandel JS. Dioxin and cancer: a critical review. Regul Toxicol Pharmacol 2003;38:378-388.ArticlePubMed
- 8. Boffetta P, Mundt KA, Adami HO, Cole P, Mandel JS. TCDD and cancer: a critical review of epidemiologic studies. Crit Rev Toxicol 2011;41:622-636.ArticlePubMedPMC
- 9. de Titto E, Savino A. Environmental and health risks related to waste incineration. Waste Manag Res 2019;37:976-986.ArticlePubMedPDF
- 10. Schuhmacher M, Meneses M, Xifró A, Domingo JL. The use of Monte-Carlo simulation techniques for risk assessment: study of a municipal waste incinerator. Chemosphere 2001;43:787-799.ArticlePubMed
- 11. Negri E, Bravi F, Catalani S, Guercio V, Metruccio F, Moretto A, et al. Health effects of living near an incinerator: a systematic review of epidemiological studies, with focus on last generation plants. Environ Res 2020;184:109305.ArticlePubMed
- 12. Porta D, Milani S, Lazzarino AI, Perucci CA, Forastiere F. Systematic review of epidemiological studies on health effects associated with management of solid waste. Environ Health 2009;8:60.ArticlePubMedPMCPDF
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-269 W64.ArticlePubMed
- 14. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467-473.ArticlePubMed
- 15. Baek K, Kwak K, Park JT. Systematic review and meta-analysis of cancer risks in relation to environmental incinerator emissions: a meta-analysis of case-control and cohort studies; 2022 [cited 2022 Jul 4]. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022292049.
- 16. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-605.ArticlePubMedPDF
- 17. Burlacu A, Artene B, Nistor I, Buju S, Jugrin D, Mavrichi I, et al. Religiosity, spirituality and quality of life of dialysis patients: a systematic review. Int Urol Nephrol 2019;51:839-850.ArticlePubMedPDF
- 18. Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:1238-1250.ArticlePubMedPMCPDF
- 19. Fowler ME, Akinyemiju TF. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int J Cancer 2017;141:2215-2227.ArticlePubMedPMCPDF
- 20. Greenland S, Thomas DC. On the need for the rare disease assumption in case-control studies. Am J Epidemiol 1982;116:547-553.ArticlePubMed
- 21. Dekkers OM, Vandenbroucke JP, Cevallos M, Renehan AG, Altman DG, Egger M. COSMOS-E: guidance on conducting systematic reviews and meta-analyses of observational studies of etiology. PLoS Med 2019;16:e1002742.ArticlePubMedPMC
- 22. Zambon P, Ricci P, Bovo E, Casula A, Gattolin M, Fiore AR, et al. Sarcoma risk and dioxin emissions from incinerators and industrial plants: a population-based case-control study (Italy). Environ Health 2007;6:19.ArticlePubMedPMCPDF
- 23. Pronk A, Nuckols JR, De Roos AJ, Airola M, Colt JS, Cerhan JR, et al. Residential proximity to industrial combustion facilities and risk of non-Hodgkin lymphoma: a case-control study. Environ Health 2013;12:20.ArticlePubMedPMCPDF
- 24. VoPham T, Bertrand KA, Jones RR, Deziel NC, DuPré NC, James P, et al. Dioxin exposure and breast cancer risk in a prospective cohort study. Environ Res 2020;186:109516.ArticlePubMedPMC
- 25. Viel JF, Clément MC, Hägi M, Grandjean S, Challier B, Danzon A. Dioxin emissions from a municipal solid waste incinerator and risk of invasive breast cancer: a population-based case-control study with GIS-derived exposure. Int J Health Geogr 2008;7:4.ArticlePubMedPMC
- 26. Ancona C, Badaloni C, Mataloni F, Bolignano A, Bucci S, Cesaroni G, et al. Mortality and morbidity in a population exposed to multiple sources of air pollution: a retrospective cohort study using air dispersion models. Environ Res 2015;137:467-474.ArticlePubMed
- 27. Ranzi A, Fano V, Erspamer L, Lauriola P, Perucci CA, Forastiere F. Mortality and morbidity among people living close to incinerators: a cohort study based on dispersion modeling for exposure assessment. Environ Health 2011;10:22.ArticlePubMedPMCPDF
- 28. Floret N, Mauny F, Challier B, Arveux P, Cahn JY, Viel JF. Dioxin emissions from a solid waste incinerator and risk of non-Hodgkin lymphoma. Epidemiology 2003;14:392-398.ArticlePubMed
- 29. Romanelli AM, Bianchi F, Curzio O, Minichilli F. Mortality and morbidity in a population exposed to emission from a municipal waste incinerator. a retrospective cohort study. Int J Environ Res Public Health 2019;16:2863.ArticlePubMedPMC
- 30. Comba P, Ascoli V, Belli S, Benedetti M, Gatti L, Ricci P, et al. Risk of soft tissue sarcomas and residence in the neighbourhood of an incinerator of industrial wastes. Occup Environ Med 2003;60:680-683.ArticlePubMedPMC
- 31. Benedetti M, Fazzo L, Guarda L, Gatti L, Comba P, Ricci P. Residential proximity to an industrial incinerator and risk of soft-tissue sarcoma, 1999-2014. Epidemiol Prev 2020;44:128-136.
- 32. Berman SH, Wandersman A. Fear of cancer and knowledge of cancer: a review and proposed relevance to hazardous waste sites. Soc Sci Med 1990;31:81-90.ArticlePubMed
- 33. Xu J, Ye Y, Huang F, Chen H, Wu H, Huang J, et al. Association between dioxin and cancer incidence and mortality: a meta-analysis. Sci Rep 2016;6:38012.ArticlePubMedPMCPDF
- 34. Kim HB, Shim JY, Park B, Lee YJ. Long-term exposure to air pollutants and cancer mortality: a meta-analysis of cohort studies. Int J Environ Res Public Health 2018;15:2608.ArticlePubMedPMC
- 35. Rezapour M, Rezapour HA, Chegeni M, Khanjani N. Exposure to cadmium and head and neck cancers: a meta-analysis of observational studies. Rev Environ Health 2021;36:577-584.ArticlePubMed
- 36. Pleus RC, Kelly KE. Health effects from hazardous waste incineration facilities: five case studies. Toxicol Ind Health 1996;12:277-287.ArticlePubMedPDF
- 37. Michelozzi P, Fusco D, Forastiere F, Ancona C, Dell’Orco V, Perucci CA. Small area study of mortality among people living near multiple sources of air pollution. Occup Environ Med 1998;55:611-615.ArticlePubMedPMC
- 38. Elliott P, Hills M, Beresford J, Kleinschmidt I, Jolley D, Pattenden S, et al. Incidence of cancers of the larynx and lung near incinerators of waste solvents and oils in Great Britain. Lancet 1992;339:854-858.ArticlePubMed
- 39. Federico M, Pirani M, Rashid I, Caranci N, Cirilli C. Cancer incidence in people with residential exposure to a municipal waste incinerator: an ecological study in Modena (Italy), 1991-2005. Waste Manag 2010;30:1362-1370.ArticlePubMed
- 40. Steenland K, Bertazzi P, Baccarelli A, Kogevinas M. Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen. Environ Health Perspect 2004;112:1265-1268.ArticlePubMedPMC
- 41. Steenland K, Deddens J, Piacitelli L. Risk assessment for 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD) based on an epidemiologic study. Am J Epidemiol 2001;154:451-458.ArticlePubMed
- 42. Pesatori AC, Consonni D, Rubagotti M, Grillo P, Bertazzi PA. Cancer incidence in the population exposed to dioxin after the “Seveso accident”: twenty years of follow-up. Environ Health 2009;8:39.ArticlePubMedPMCPDF
- 43. Catalani S. IARC revision on dioxin and some dioxin-like compounds. G Ital Med Lav Ergon 2010;32:79-81 (Italian).
- 44. McGregor DB, Partensky C, Wilbourn J, Rice JM. An IARC evaluation of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans as risk factors in human carcinogenesis. Environ Health Perspect 1998;106(Suppl 2):755-760.ArticlePubMedPMC
- 45. Pesatori AC, Consonni D, Bachetti S, Zocchetti C, Bonzini M, Baccarelli A, et al. Short- and long-term morbidity and mortality in the population exposed to dioxin after the “Seveso accident”. Ind Health 2003;41:127-138.ArticlePubMed
- 46. Warner M, Eskenazi B, Mocarelli P, Gerthoux PM, Samuels S, Needham L, et al. Serum dioxin concentrations and breast cancer risk in the Seveso Women’s Health Study. Environ Health Perspect 2002;110:625-628.ArticlePubMedPMC
- 47. Li Z. Risk-based principles and incompleteness theorems for linear dose-response extrapolation for carcinogenic chemicals. Chemosphere 2020;247:125934.ArticlePubMed
- 48. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. IARC Monogr Eval Carcinog Risks Hum 1993;58:1-415.PubMedPMC
- 49. Xie R, Li WJ, Li J, Wu BL, Yi JQ. Emissions investigation for a novel medical waste incinerator. J Hazard Mater 2009;166:365-371.ArticlePubMed
- 50. Cangialosi F, Intini G, Liberti L, Notarnicola M, Stellacci P. Health risk assessment of air emissions from a municipal solid waste incineration plant—a case study. Waste Manag 2008;28:885-895.ArticlePubMed
- 51. Bena A, Gandini M, Cadum E, Procopio E, Salamina G, Orengia M, et al. Risk perception in the population living near the Turin municipal solid waste incineration plant: survey results before start-up and communication strategies. BMC Public Health 2019;19:483.ArticlePubMedPMCPDF
- 52. Petts J. Incineration risk perceptions and public concern: experience in the UK improving risk communication. Waste Manag Res 1992;10:169-182.ArticlePDF
- 53. Goldberg MS. On the interpretation of epidemiological studies of ambient air pollution. J Expo Sci Environ Epidemiol 2007;17 Suppl 2:S66-S70.ArticlePubMedPDF
- 54. Boffetta P. Human cancer from environmental pollutants: the epidemiological evidence. Mutat Res 2006;608:157-162.ArticlePubMed
- 55. Goria S, Daniau C, de Crouy-Chanel P, Empereur-Bissonnet P, Fabre P, Colonna M, et al. Risk of cancer in the vicinity of municipal solid waste incinerators: importance of using a flexible modelling strategy. Int J Health Geogr 2009;8:31.ArticlePubMedPMC
- 56. Quass U, Fermann M, Bröker G. The European dioxin air emission inventory project—final results. Chemosphere 2004;54:1319-1327.ArticlePubMed
- 57. McKay G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: review. Chem Eng J 2002;86:343-368.Article
- 58. Dopico M, Gómez A. Review of the current state and main sources of dioxins around the world. J Air Waste Manag Assoc 2015;65:1033-1049.ArticlePubMed
- 59. Shafran-Nathan R, Levy I, Levin N, Broday DM. Ecological bias in environmental health studies: the problem of aggregation of multiple data sources. Air Qual Atmos Health 2017;10:411-420.ArticlePDF
- 60. Santibáñez-Andrade M, Quezada-Maldonado EM, Osornio-Vargas Á, Sánchez-Pérez Y, García-Cuellar CM. Air pollution and genomic instability: the role of particulate matter in lung carcinogenesis. Environ Pollut 2017;229:412-422.ArticlePubMed
- 61. Hong YC, Lee KH, Kwon HJ, Jang JY, Leem JH. Exposure assessment of PCDD/Fs and monitoring of health effects on workers and resident near the waste incinerators in Korea. J Prev Med Public Health 2003;36:314-322 (Korean).
- 62. Allsopp M, Costner P, Johnston P. Incineration and human health. State of knowledge of the impacts of waste incinerators on human health. Environ Sci Pollut Res Int 2001;8:141-145.PubMed
- 63. Committee on Health Effects of Waste Incineration; Board on Environmental Studies and Toxicology; Commission on Life Sciences; National Research Council. Waste incineration and public health. Washington, DC: National Academies Press; 2000. p 112-181.
REFERENCES
Figure & Data
References
Citations


 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite