Articles
- Page Path
- HOME > Epidemiol Health > Volume 45; 2023 > Article
-
COVID-19
Cohort Profile
Cohort profile: investigating SARS-CoV-2 infection and the health and psychosocial impact of the COVID-19 pandemic in the Canadian CHILD Cohort -
Rilwan Azeez1,2
 , Larisa Lotoski2,3
, Larisa Lotoski2,3 , Aimée Dubeau4,5
, Aimée Dubeau4,5 , Natalie Rodriguez2,3
, Natalie Rodriguez2,3 , Myrtha E. Reyna4
, Myrtha E. Reyna4 , Tyler Freitas5
, Tyler Freitas5 , Stephanie Goguen2,3
, Stephanie Goguen2,3 , Maria Medeleanu4
, Maria Medeleanu4 , Geoffrey L. Winsor6
, Geoffrey L. Winsor6 , Fiona S. L. Brinkman6
, Fiona S. L. Brinkman6 , Emily E. Cameron7
, Emily E. Cameron7 , Leslie Roos2,7
, Leslie Roos2,7 , Elinor Simons2,3
, Elinor Simons2,3 , Theo J. Moraes4
, Theo J. Moraes4 , Piush J. Mandhane8
, Piush J. Mandhane8 , Stuart E. Turvey9
, Stuart E. Turvey9 , Shelly Bolotin10
, Shelly Bolotin10 , Kim Wright5
, Kim Wright5 , Deborah McNeil11,12
, Deborah McNeil11,12 , David M. Patrick13,14
, David M. Patrick13,14 , Jared Bullard2,15
, Jared Bullard2,15 , Marc-André Langlois16
, Marc-André Langlois16 , Corey R. Arnold16
, Corey R. Arnold16 , Yannick Galipeau16, Martin Pelchat16
, Yannick Galipeau16, Martin Pelchat16 , Natasha Doucas17, Padmaja Subbarao4,5
, Natasha Doucas17, Padmaja Subbarao4,5 , Meghan B. Azad1,2,3
, Meghan B. Azad1,2,3
-
Epidemiol Health 2023;45:e2023091.
DOI: https://doi.org/10.4178/epih.e2023091
Published online: October 13, 2023
1Department of Immunology, University of Manitoba, Winnipeg, MB, Canada
2Children’s Hospital Research Institute of Manitoba, Winnipeg, MB, Canada
3Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, MB, Canada
4Division of Respiratory Medicine, Department of Pediatrics and Program in Translational Medicine, SickKids Research Institute, The Hospital for Sick Children, Toronto, ON, Canada
5Department of Medicine, Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada
6Department of Molecular Biology and Biochemistry, Simon Fraser University, Burnaby, BC, Canada
7Department of Psychology, University of Manitoba, Winnipeg, MB, Canada
8Department of Pediatrics, University of Alberta, Edmonton, AB, Canada
9Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada
10Centre for Vaccine Preventable Diseases, University of Toronto; Dalla Lana School of Public Health, University of Toronto; Department of Laboratory Medicine and Pathobiology, University of Toronto; Public Health Ontario, ON, Canada
11Department of Community Health Sciences, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
12Strategic Clinical Networks, Alberta Health Services, Calgary, AB, Canada
13School of Population and Public Health, University of British Columbia, Vancouver, BC, Canada
14British Columbia Centre for Disease Control, Vancouver, BC, Canada
15Cadham Provincial Laboratory, Manitoba Health, Winnipeg, MB, Canada
16Department of Biochemistry, Microbiology and Immunology, University of Ottawa, Ottawa, ON, Canada
17CHILD Cohort Study National Parent Engagement Committee
- Correspondence: Meghan B. Azad Department of Pediatrics and Child Health, University of Manitoba, 501G-715 McDermot Avenue, Winnipeg, MB R3M 3P4, Canada E-mail: meghan.azad@umanitoba.ca
© 2023, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,347 Views
- 91 Download
Abstract
- The coronavirus disease 2019 (COVID-19) pandemic has affected all Canadian families, with some impacted differently than others. Our study aims to: (1) determine the prevalence and transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among Canadian families, (2) identify predictors of infection susceptibility and severity of SARS-CoV-2, and (3) identify health and psychosocial impacts of the COVID-19 pandemic. This study builds upon the CHILD Cohort Study, an ongoing multi-ethnic general population prospective cohort consisting of 3,454 Canadian families with children born in Vancouver, Edmonton, Manitoba, and Toronto between 2009 and 2012. During the pandemic, CHILD households were invited to participate in the CHILD COVID-19 Add-On Study involving: (1) brief biweekly surveys about COVID-19 symptoms and testing; (2) quarterly questionnaires assessing COVID-19 exposure and testing, vaccination status, physical and mental health, and pandemic-driven life changes; and (3) in-home biological sampling kits to collect blood and stool. In total, 1,462 households (5,378 participants) consented to the CHILD COVID-19 Add-On Study: 2,803 children (mean±standard deviation [SD], 9.0±2.7 years; range, 0-17 years) and 2,576 adults (mean±SD, 43.0±6.5 years; range, 18-85 years). We will leverage the wealth of pre-pandemic CHILD data to identify risk and resilience factors for susceptibility and severity to the direct and indirect pandemic effects. Our short-term findings will inform key stakeholders and knowledge users to shape current and future pandemic responses. Additionally, this study provides a unique resource to study the long-term impacts of the pandemic as the CHILD Cohort Study continues.
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection results in a broad range of clinical phenotypes, from asymptomatic infection to severe disease and death [1,2]. Pre-existing medical conditions, socioeconomic disadvantage, occupation, gender, and race/ethnicity have been associated with SARS-CoV-2 infection rates and disease severity in adults [3-6]. However, much of the biological variation in this new disease remains unexplained, and more research is needed to understand why children are less likely than adults to develop severe coronavirus disease 2019 (COVID-19) [7-9]. Identifying these divergent outcomes in adults and children will help inform prevention and treatment strategies for people of all ages [10]. Additionally, understanding the persistence and biology of antibody responses following infection or vaccination will help inform public health responses to the ongoing pandemic.
- In an effort to control this pandemic, public health measures have varied across Canada both geographically and over time. COVID-19 vaccination and non-pharmaceutical interventions, such as masking and school closures, have helped slow the spread of COVID-19 in Canada and beyond [3,11,12]. However, these measures have had consequences for the economy, mental health, and emotional and physical well-being of family members. They may also amplify inequities experienced by marginalized and vulnerable populations [12]. It is therefore important to assess how pandemic control measures differentially affect the short-term and long-term physical and mental health of Canadian families.
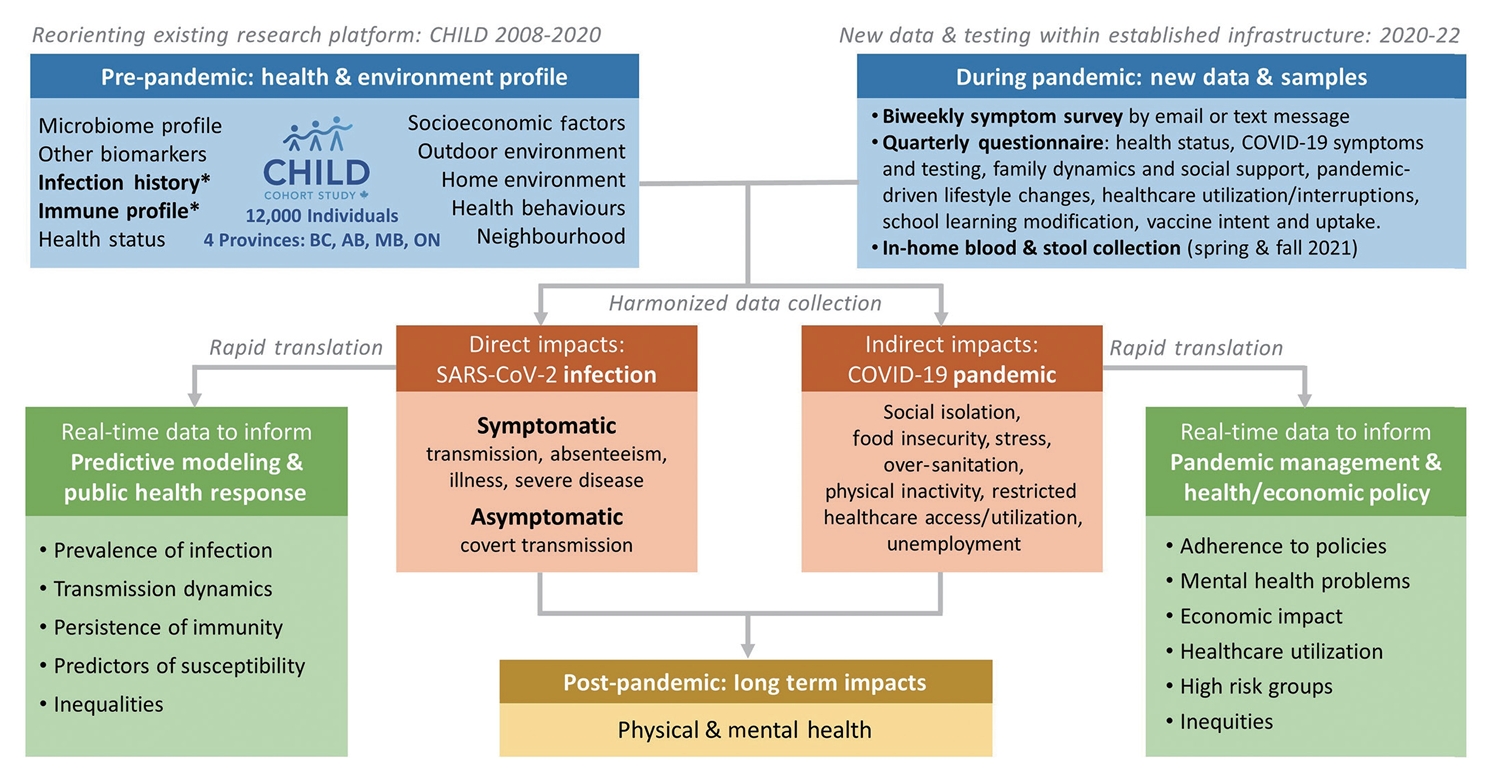
- The CHILD Cohort Study (www.childstudy.ca), an ongoing birth cohort of 3,454 families, offers a unique opportunity to study COVID-19, as the CHILD Cohort Study has collected a wealth of socio-demographic, health, and biological data since 2009. The CHILD COVID-19 Add-On Study (Figure 1) will investigate (1) symptomatic and asymptomatic SARS-CoV-2 infection prevalence, transmission, and immunity; (2) predictors of SARS-CoV-2 infection susceptibility and severity; (3) prevalence and predictors of health and psychosocial impacts of the COVID-19 pandemic on CHILD families; and (4) individual and social determinants of COVID-19 vaccine uptake or hesitancy. Furthermore, it will provide a lasting resource to study the long-term impacts of the pandemic as the CHILD Cohort Study continues.
INTRODUCTION
- Study design and population
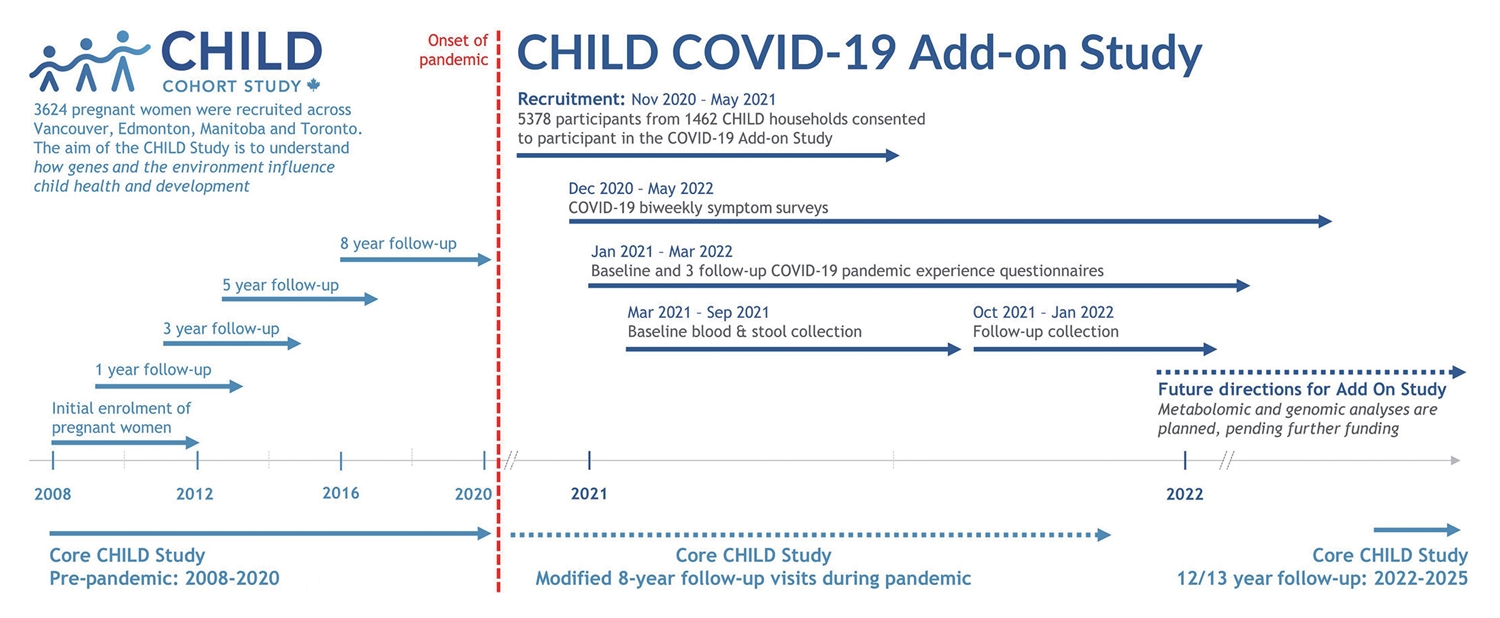
- This is a prospective longitudinal study embedded in the existing CHILD cohort, which recruited 3,454 families (~10,000 individuals) from the general population with children born in British Columbia, Alberta, Manitoba, and Ontario between 2009 and 2012 (90.7% retention to date) [13]. The eligible participants for the COVID Add-On Study included all parents and children enrolled in the CHILD Cohort Study, along with all other individuals living in the same household (n=3,032 eligible households) (Figure 2). This COVID Add-On Study involves (1) brief biweekly surveys about COVID-19 symptoms and testing; (2) quarterly questionnaires assessing COVID-19 exposure and vaccination status and pandemic-driven life changes; (3) in-home collection of blood for SARS-CoV-2 immunoglobulin G serology, biomarker analysis, and (optionally) genetic analysis, and stool for microbiome analysis.
- Consent, recruitment, and retention
- Eligible participants were invited to take part in this study during in-person CHILD Cohort Study clinic visits or by phone, video call, or e-mail, between November 2020 and May 2021. E-mail invitations included a summary of the study, a YouTube video (https://www.youtube.com/watch?v=lEzbn7HYiQw) describing the study’s purpose and participation requirements, and instructions on how to arrange a virtual consent appointment with research staff. Participants attending clinic visits were provided paper consent forms. Otherwise, consent was obtained virtually during a videoconference appointment with study staff (Zoom; Zoom Video Communications, Inc., San Jose, CA, USA) using the web browser-based Research Electronic Data Capture (REDCap) consent module [14,15]. Child participant assent (as defined provincially by age) or consent was obtained in the same manner in the presence of their caregiver. Participants were invited to additionally provide consent for 3 optional activities: (1) whole genome sequencing (65.1% consented), (2) future undefined analyses of their biological samples (69.4%), and (3) analysis of previously collected biological samples for future unknown research studies (59.4%). To encourage retention, we provided gift cards (US$25/household for completion of the baseline survey, and again for return of biological samples) and returned SARS-CoV-2 serology results to participants.
- Biweekly surveys
- Study participants’ COVID-19 symptoms, exposure and diagnosis were captured through a biweekly symptom survey. Beginning in December 2020, 1 designated participant per household was prompted biweekly by text message or e-mail (according to their preference) with 3 COVID-19 screening questions asking if anyone in their household had symptoms of, was suspected of having, or was tested for COVID-19. Participants responding “yes” to any of these questions were sent a follow-up COVID-19 symptom survey by e-mail to collect individual-level information on specific COVID-19 symptoms and testing, travel, and exposure history, as well as clinical or public health action taken after a positive COVID-19 test result. Completion rates for the biweekly surveys ranged from 70.0% in the Edmonton site to 88% in the Winnipeg site and were generally consistent over time.
- Quarterly questionnaires
- Questionnaires (Supplementary Material 1) were delivered online using REDCap at enrolment and approximately every 3 months thereafter. They were adapted from other Canadian COVID-19 studies, and addressed the following topics: individual demographic characteristics, employment status, COVID-19 pandemic government support use, mental and physical health, school closures, adherence to non-pharmaceutical public health measures, screen time and use of technology for school/work or other purposes, COVID-19 symptoms, testing and diagnosis, travel history and COVID-19 vaccination perceptions and status (Table 1). There were 3 versions of each questionnaire, designed for completion by children (about themselves), adults (about themselves), or parents/caregivers (about their children). Individuals able to provide consent on their own behalf were asked to complete the adult questionnaire. All remaining participants (i.e., children enrolled through assent or caregiver consent) were asked to complete the child questionnaire. The caregivers of participating children were also asked to complete a parent questionnaire for each child enrolled in their household. The completion rates for the quarterly questionnaires ranged from 84.9% (n=6,287) at baseline to 43.9% (n=3,248) at the final follow-up (Table 2). In general, the socio-demographic characteristics of participants who completed the final follow-up survey (51.3% of adults and 49.0% of children) did not differ much from those who did not. However, adult participants who did not complete the final survey were slightly more likely to have a lower level of formal education (Supplementary Material 2).
- In-home biological sample collection and severe acute respiratory syndrome coronavirus 2 serology
- Blood and stool samples were collected twice (spring and fall/winter 2021) using in-home sampling kits sent to each household along with a pre-paid return shipment box. Participants were asked to collect all samples within a 48-hour period of receipt. Sample kits were prepared by the DaklaPack company (Moonachie, NJ, USA) and included all materials required to collect blood and stool, including an instructional pamphlet (Supplementary Material 3), 2-ply 70% alcohol swabs, 2”× 2” 8-ply sterile gauze sponges (CA95041-740; VWR, Radnor, PA, USA), bandages, a registration card and specimen bag containing a Desco Humidity Indicator Cards (89131-360; VWR International) and MiniPax absorbent desiccant packets (Z163570; Sigma-Aldrich, St. Louis, MO, USA). Sample packages were returned to the Clinical Research Laboratory and Biobank (CRLB, Hamilton, ON, Canada) by airmail. The collection rates for biological samples were 56.4% in the first wave and 57.0% in the second wave of collection (Table 2).
- Blood specimens were collected using the dried blood spot (DBS) method and Mitra Blood Collection devices. DBS samples were collected using a Whatman 903 protein saver 5-spot card (VWR International). Returned sample bags containing DBS collection cards, desiccant pouches, and humidity cards were sealed in a second polybag and stored at 4°C for SARS-CoV-2 serological analysis using automated chemiluminescent enzyme-linked immunosorbent assays to detect anti-spike and anti-nucleoprotein antibodies as described by Cholette et al. [16]. COVID-19 antigens and the anti-hIgG#5-HRP fusion antibody were generously provided by Dr. Yves Durocher, National Research Council of Canada. The cut-offs for seropositivity used were based on false discovery rates in pre-pandemic cohorts of 2% for the spike and both 1% and 10% for the nucleoprotein. Preserved blood was also collected using Mitra clamshell devices containing 2 samplers (Neoteryx, Torrance, CA, USA). Upon receipt, the clamshell casing was removed, samplers were loaded into a 96-autorack, placed into a polybag containing a desiccant pouch, and stored at -80°C until further use. Metabolomic and genomic analyses are planned, pending further funding.
- Stool samples were collected by participants using 2 cottontipped swabs. Fecal swabs were then suspended in a capped collection tube containing ethanol for transport. Upon receipt, the shafts of the fecal swabs were cut off and the specimens were cryopreserved. Microbiome analyses are planned, pending further funding.
- Statistical analysis plan
- The prevalence and severity of infection will be estimated from self-reported biweekly reports, quarterly questionnaire testing information, and serological testing. Severity will be classified as asymptomatic, mild, or severe using World Health Organization criteria [17]. Transmission will be assessed using multilevel modelling (by household and study site). Persistence of SARS-CoV-2-induced immunity will be determined from longitudinal serology testing for SARS-CoV-2 antibodies over 2 time frames (spring and fall 2021).
- We will combine new data from the CHILD COVID-19 Add-On Study with existing pre-pandemic CHILD data to predict SARS-CoV-2 infection susceptibility (incidence) and severity. We hypothesize that infection susceptibility will be associated with (1) physical interactions with (or distancing from) other individuals in the community during the pandemic; (2) socio-demographic characteristics including sex, age, occupation, socioeconomic status, and household size; (3) pre-existing medical conditions including obesity and asthma; (4) neighbourhood characteristics including air pollution exposure, housing type, green and blue spaces; and (5) pre-pandemic immune biomarker and genetic profiles, viral infection history (including pre-existing coronavirus serology), respiratory health (wheezing, lung function), and health-related lifestyle factors (e.g., diet quality and physical activity). Associations will be tested in multivariate regression models with a 3-category outcome (symptomatic, asymptomatic, or no infection), random forest analysis, gradient boosting, recursive feature elimination and mutual information method. If sufficient cases are identified, we will also evaluate infection severity.
- We will quantify the prevalence of and changes between prepandemic and during-pandemic physical and mental health functioning (not directly related to SARS-CoV-2 infection). Prediction models, as described for aim 2, will be used to identify factors that predict risk for (or protection against) poor pandemic-related mental and physical health functioning. We hypothesize that protective factors will include (1) maintaining social connections, physical activity, healthy eating behaviours, and adequate sleep during the pandemic; (2) higher pre-pandemic mental well-being; and (3) continued employment throughout the pandemic.
- Ethics statement
- This study was approved by research ethics boards at the University of British Columbia (H20-02324), University of Alberta (Pro00102524), University of Manitoba (HS24250), The Hospital for Sick Children (1000071220), and McMaster University (1108).
COHORT DESCRIPTION
Blood collection
Stool collection
Aim 1: estimate SARS-CoV-2 infection, transmission, severity, and immunity
Aim 2: identify predictors of SARS-CoV-2 infection susceptibility and severity
Aim 3: understand the impact of the COVID-19 pandemic on the mental and physical health of Canadian families using a health equity lens
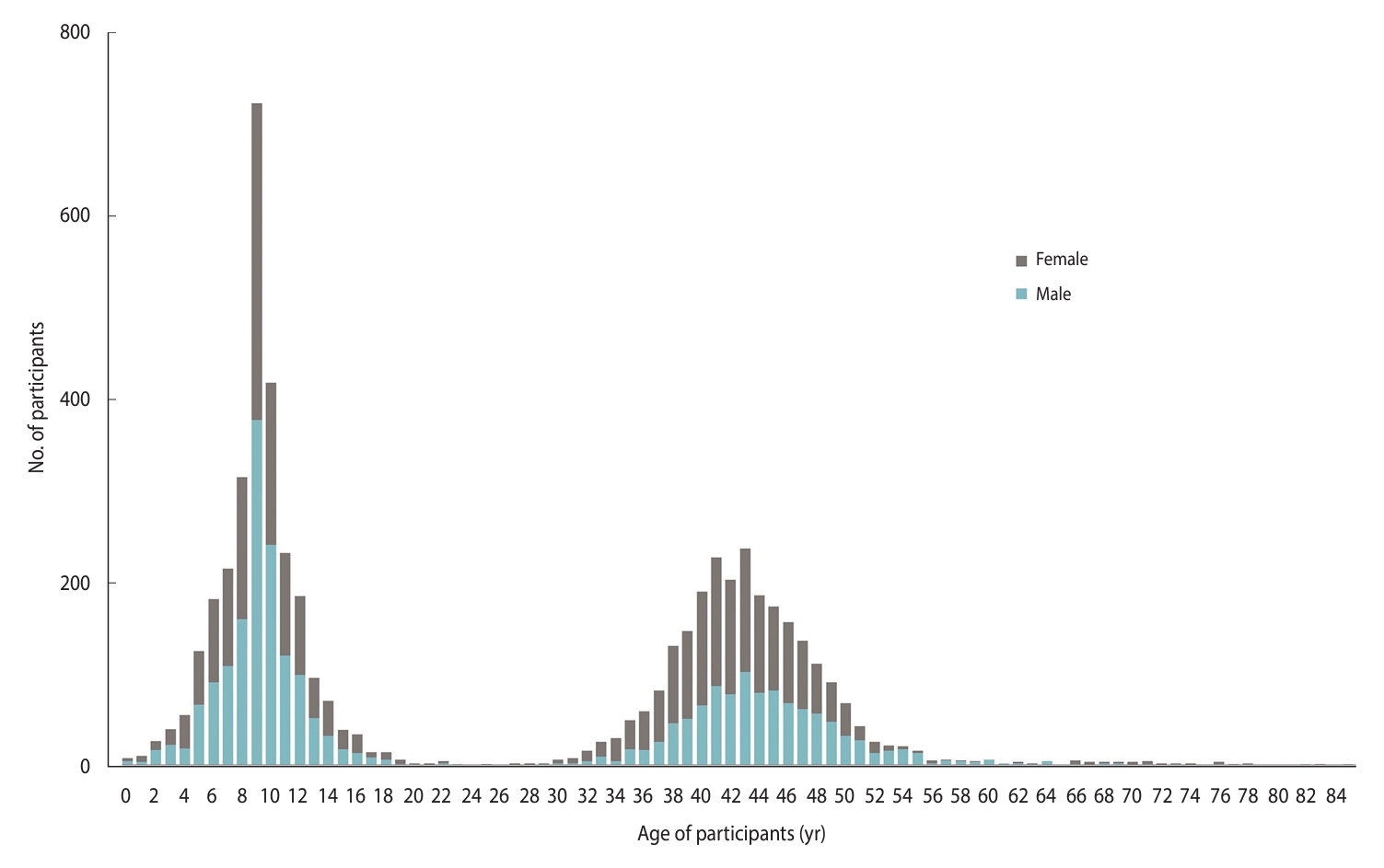
- The final CHILD COVID-19 Add-On Study population included 5,378 participants from 1,462 households (mean, 3.7 participants per household; interquartile range [IQR], 3-4; range, 1-12), of whom 3,848 were original CHILD participants (1,431 mothers, 1,015 fathers, and 1,402 children). The remaining 1,530 participants were siblings (n = 1,427) or other household members (n=103). Almost half (48%) of the eligible CHILD households consented to participate in the CHILD COVID-19 Add-On Study. A total of 370 households (12% of those eligible) actively declined to participate, while the remainder were unresponsive (40%, n=1,205). Compared to non-participating CHILD families, those participating in the COVID-19 Add-On Study tended to have higher education and household income, and larger households (Supplementary Material 4). Participants were distributed across the Manitoba (34%), Vancouver (27%), Toronto (22%), and Edmonton (17%) study sites. Among children enrolled (n=2,802, 52% female), the mean age was 9.0 years (standard deviation [SD], 2.7; range, 0-17), while among adults (n = 2,576, 58% female), the mean age was 43.0 years (SD, 6.5; range, 18-85) (Figure 3). Participants predominantly identified as having European ancestral origins (77%), and the majority (68%) of adults had a university degree.
- At enrolment, 31% of adults were essential workers (e.g., healthcare, delivery, store, security, and building maintenance), with proportions ranging from 17% in Toronto to 44% in Manitoba. During the first wave of the pandemic, 45% of working adults moved to remote work, 9% lost their job (permanently or temporarily), and 35% accessed government supports (e.g., loan deferrals, personal or business income support). The majority of children (84%) experienced school closures during spring 2020 (range, 80-89% across study sites), of which 23% returned to in-person classes before the end of the academic year in June 2020 (ranging from 6% in Edmonton to 51% in Vancouver). Most children who experienced school closures were offered (92%) and attended (85% fully and 13% partially) online classes (Table 3).
- Patient and public involvement statement
- The CHILD COVID-19 Add-On Study adopts an integrated knowledge translation approach. To accelerate the availability and translation of high-quality, real-time evidence, we included key stakeholders on our team to develop the study and translate and disseminate results. Our team includes members of the CHILD Parent Advisory Committee and Knowledge Users from public health authorities at the national (Public Health Agency of Canada) and provincial levels (BC Centre for Disease Control, Alberta Health Services, Manitoba Shared Health, Public Health Ontario) who meet monthly with study investigators to share data in real time and adapt procedures if necessary (e.g., modify symptom surveys, questionnaires, and serology testing methods) to ensure that our results can be rapidly translated into policy and practice. The study investigators also meet monthly with the Canadian Covid Immunity Task Force Pediatric Working Group to share experiences and expertise, develop harmonized protocols, and foster research collaborations. A Rapid Results website (https://childstudy.ca/covid-rapid-results/) was developed to facilitate rapid widespread translation of study results to participants and stakeholders. To maximize recruitment, retention, and relevance to families, the CHILD Parent Advisory Committee was engaged to co-develop the study design, grant application, recruitment strategies, and questionnaires.
- In addition to traditional dissemination of results through open access publication in peer-reviewed journals, we will use our website (childstudy.ca/covid-rapid-results) and social media (Twitter) to rapidly disseminate time-sensitive findings that are relevant to stakeholders and knowledge users, including provincial public health organizations and non-profit organizations (e.g., Children First Canada). We will also engage the CHILD Knowledge Mobilization Stakeholder Advisory Committee, which includes stakeholders from government, industry, patient organizations, clinical societies, child health-focused organizations, parenting-focused communications platforms, and CHILD parents.
KEY FINDINGS
- Our cohort offers a unique opportunity to study the clinical features and long-term sequelae of COVID-19, assess viral transmission within households, understand COVID-19 vaccine uptake/hesitancy, and investigate the psychosocial impact of the pandemic and its management among Canadian children and their families. The CHILD cohort has rich pre-pandemic longitudinal data and biosamples available to investigate how current and pre-pandemic health status, immune phenotypes and biomarkers can interactively predict susceptibility and resilience to COVID-19 and the psychosocial impacts of the pandemic.
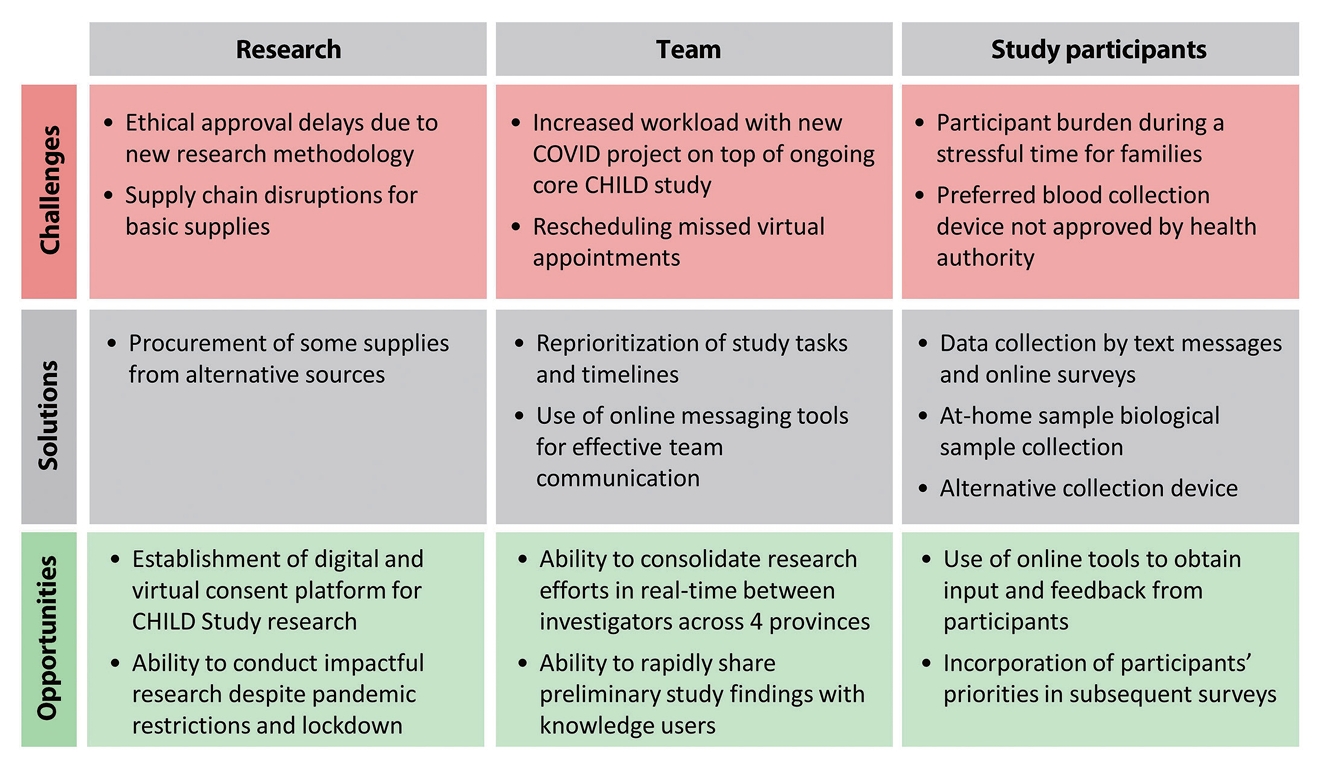
- Unanticipated challenges encountered during the pandemic delayed data collection during a critical period of the pandemic (summer 2020). For example, ethical approvals were delayed by the introduction of many novel study elements (e.g., virtual consent by video conference and in-home sample collection). Pandemic-driven supply chain disruptions made acquiring biological sample kit components (e.g., gauze and bandages) exceedingly difficult, leading to delays in sample collection. Despite being used in the United States for serology testing, the Mitra Clamshell devices were not approved by Health Canada in time for study deployment. Standard DBS methods were used instead, requiring a more painful finger prick and larger blood volume, which negatively impacted retention (Figure 4).
- Generalizability may be limited by selection bias because socioeconomic status tended to be higher among CHILD families than in the general Canadian population. Further, we noted significant differences in socio-demographic characteristics (e.g., parental education and total household income) between participating and non-participating CHILD households. This selection bias may have excluded the most vulnerable populations during the precarious early pandemic period, further exacerbating the lack of generalizability of this cohort. Sensitivity analyses may be conducted to explore the robustness of study findings where applicable. The frequent biweekly questionnaires and lengthy adult and parent quarterly questionnaires created a large burden on the designated household participant responsible for providing responses for themselves and all household members. Collectively, the high burden placed on participants led to study withdrawal in a subset of families.
- To our knowledge, there is no other multi-site pediatric cohort with rich longitudinal data and recent pre-pandemic biosamples available to investigate how current and pre-pandemic health status, immune phenotypes, and biomarkers can interaactively predict susceptibility and resilience to COVID-19 and the psychosocial impacts of the pandemic. The CHILD Cohort offers a unique and powerful opportunity to study the clinical features and longterm sequelae of COVID-19, assess viral transmission within households, understand COVID-19 vaccine uptake/hesitancy, and investigate the psychosocial impact of the pandemic and its management among Canadian children and their families.
STRENGTHS AND WEAKNESSES
- Data are available upon reasonable request. Researchers interested in developing or collaborating on a project using CHILD data are encouraged to contact the study’s National Coordinating Centre for a formal request. Details for data request and access are outlined on the CHILD website: https://childstudy.ca/for-researchers/study-data/.
DATA ACCESSIBILITY
SUPPLEMENTARY MATERIALS
Supplement Material 1.
Supplement Material 2.
-
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare for this study.
-
FUNDING
This work was supported by funding from the Canadian Institutes of Health Research, the Canadian COVID-19 Immunity Task Force [VR5-172658], and Research Manitoba [4494]. Core funding for the CHILD Cohort Study was provided by the Canadian Institutes of Health Research [CIHR; AEC-85761, PJT-148484, FDN-159935, and EC1-144621], the Allergy, Genes and Environment Network of Centres of Excellence (AllerGen NCE) [12CHILD], BC Children’s Hospital Foundation, Don & Debbie Morrison, and Genome Canada/Genome BC [274CHI]. This research was supported, in part, by the Canada Research Chairs program: MBA holds a Tier 2 Canada Research Chair in the Developmental Origins of Health and Disease; SET holds a Tier 1 Canada Research Chair in Pediatric Precision Health; PS holds a Tier 1 Canada Research Chair in Pediatric Asthma & Lung Health. EC is supported by a Social Science and Humanities Research Council Postdoctoral Fellowship. FSLB is an SFU Distinguished Professor. YG is supported by a Frederick Banting and Charles Best CGS-D from the Canadian Institutes of Health Research [FBD-181487]. Production of COVID-19 reagents was financially supported by NRC’s Pandemic Response Challenge Program. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. In alignment with the call from the Canadian Chief Science Advisors, this COVID-19-related publication will be open access.
-
AUTHOR CONTRIBUTIONS
Conceptualization: Azeez R, Lotoski L, Dubeau A, Brinkman FSL, Bolotin S, McNeil D, Wright K, Bullard J, Patrick DM, Simons E, Moraes TJ, Turvey SE, Mandhane PJ, Doucas N, Subbarao P, Rodriguez N, Azad MB. Data curation: Dubeau A, Freitas T, Winsor GL, Goguen S, Brinkman FSL. Formal analysis: Azeez R, Reyna ME, Winsor GL, Goguen S, Brinkman FSL, Azad MB. Funding acquisition: Brinkman FSL, Bolotin S, McNeil D, Wright K, Bullard J, Patrick DM, Simons E, Moraes TJ, Turvey SE, Mandhane PJ, Subbarao P, Rodriguez N, Azad MB. Methodology: Azad MB. Project administration: Lotoski L, Dubeau A, Freitas T, Rodriguez N, Azad MB. Visualization: Azeez R. Writing – original draft: Azeez R, Lotoski L, Medeleanu M, Azad MB. Writing – reviewing & editing: Azeez R, Lotoski L, Medeleanu M, Azad MB, Reyna ME, Brinkman FSL, Langlois MA, Arnold CR, Galipeau Y, Pelchat M, Bolotin S, McNeil D, Wright K, Bullard J, Patrick DM, Simons E, Moraes TJ, Turvey SE, Mandhane PJ, Subbarao P, Doucas N, Rodriguez N, Dubeau A, Freitas T, Winsor GL, Goguen S, Cameron EE, Roos L, Reyna ME, Freitas T.
NOTES
ACKNOWLEDGEMENTS
| Questionnaires |
Baseline |
Follow-up 1 |
Follow-up 2 |
Follow-up 3 |
|---|---|---|---|---|
| Collection period | Jan-Jun, 2021 | Aug-Sep, 2021 | Oct-Dec, 2021 | Jan-Mar, 2022 |
| Completion rate by participant type | ||||
| Child self-report | 1,643/2,064 (79.6) | 987/2,064 (47.8) | 880/2,064 (42.6) | 794/2,064 (38.5) |
| Parent on behalf of child | 2,336/2,766 (84.4) | 1,527/2,766 (55.2) | 1,275/2,766 (46.1) | 1,202/2,766 (43.5) |
| Adult self-report | 2,308/2,576 (89.6) | 1,563/2,576 (60.7) | 1,370/2,576 (53.2) | 1,252/2,576 (48.6) |
| Overall completion rate | 6,287/7,406 (84.9) | 4,077/7,406 (55.0) | 3,525/7,406 (47.6) | 3,248/7,406 (43.9) |
| Biological samples |
Phase A (n=5,326) |
Phase B (n=3,219)1 |
||
| Collection period | Mar-Sep, 2021 | Oct-Dec, 2021 | ||
| Sample type | Stool | Blood | Stool | Blood |
| Children | 1,445/2,738 (52.8) | 1,462/2,738 (53.4) | 823/1,648 (49.9) | 847/1,648 (51.4) |
| Adults | 1,506/2,588 (58.2) | 1,567/2,588 (60.5) | 969/1,571 (61.7) | 1,022/1,571 (65.0) |
| Total | 2,951/5,326 (55.4) | 3,029/5,326 (56.9) | 1,792/3,219 (55.6) | 1,869/3,219 (58.1) |
| Characteristics |
Study site |
Total | |||
|---|---|---|---|---|---|
| Vancouver | Edmonton | Manitoba | Toronto | ||
| Households | |||||
| Eligible for study | 700 (100) | 687 (100) | 937 (100) | 709 (100) | 3,033 (100) |
| Consented for study (% of eligible) | 407 (58.1) | 308 (44.8) | 447 (47.7) | 300 (42.3) | 1,462 (48.2) |
| Individuals enrolled (% of total) | 1,468 (27.3) | 915 (17.0) | 1,800 (33.5) | 1,195 (22.2) | 5,378 (100) |
| Mothers from original CHILD Study | 398 | 300 | 442 | 291 | 1,431 (26.6) |
| Fathers from original CHILD Study | 292 | 132 | 354 | 237 | 1,015 (18.9) |
| Children from original CHILD Study | 399 | 268 | 441 | 294 | 1,402 (26.1) |
| Siblings | 352 | 203 | 521 | 351 | 1,427 (26.5) |
| Other household members | 27 | 12 | 42 | 22 | 103 (1.9) |
| Ancestry (participants, N)1 | 1,458 | 909 | 1,779 | 1,194 | 5,340 |
| North America (not First Nations) | 124 (8.5) | 119 (13.1) | 185 (10.4) | 90 (7.5) | 518 (9.7) |
| First Nations | 25 (1.7) | 62 (6.8) | 178 (10.0) | 19 (1.6) | 284 (5.3) |
| UK or Europe | 1,012 (69.4) | 662 (72.8) | 1,332 (74.9) | 789 (66.1) | 3,795 (71.1) |
| Central or South America | 29 (2.0) | 23 (2.5) | 44 (2.5) | 55 (4.6) | 151 (2.8) |
| Africa | 20 (1.4) | 10 (1.1) | 24 (1.3) | 50 (4.2) | 104 (1.9) |
| Middle East | 25 (1.7) | 13 (1.4) | 5 (0.3) | 36 (3.0) | 79 (1.5) |
| Asia or Polynesia | 251 (17.2) | 81 (8.9) | 125 (7.0) | 221 (18.5) | 678 (12.7) |
| Australia or New Zealand | 34 (2.3) | 5 (0.6) | 6 (0.3) | 6 (0.5) | 51 (0.9) |
| Educational attainment (adults, N) | 600 | 378 | 729 | 471 | 2,178 |
| High school or less | 14 (2.3) | 30 (7.9) | 113 (15.5) | 29 (6.2) | 186 (8.5) |
| Postsecondary certificate/diploma | 113 (18.8) | 124 (32.8) | 217 (29.8) | 48 (10.2) | 502 (23.0) |
| Bachelor’s degree | 243 (40.5) | 162 (42.8) | 268 (36.8) | 226 (48.0) | 899 (41.3) |
| Graduate degree | 230 (38.3) | 62 (16.4) | 131 (18.0) | 168 (35.7) | 591 (27.1) |
| Occupation (adults, N) | 597 | 375 | 730 | 479 | 2,181 |
| Working | 497 (83.2) | 303 (80.8) | 638 (87.4) | 394 (82.2) | 1,832 (84.0) |
| Not working2 | 100 (16.7) | 72 (19.2) | 92 (12.6) | 85 (17.7) | 349 (16.0) |
| Occupation type (adults, N) | 563 | 354 | 667 | 458 | 2,042 |
| Essential worker3 | 142 (25.2) | 112 (31.6) | 294 (44.1) | 80 (17.5) | 628 (30.7) |
| Pandemic impact on work (adults, N)1 | 616 | 393 | 762 | 499 | 2,270 |
| Moved to remote work | 306 (49.7) | 148 (37.6) | 277 (36.3) | 281 (56.3) | 1,012 (44.6) |
| Lost job, permanently | 10 (1.6) | 12 (3.0) | 16 (2.1) | 13 (2.6) | 51 (2.2) |
| Lost job, temporarily | 42 (6.8) | 31 (7.9) | 47 (6.2) | 28 (5.6) | 148 (6.5) |
| Got new job | 31 (5.0) | 18 (4.6) | 33 (4.3) | 19 (3.8) | 101 (4.4) |
| Reduced work hours | 82 (13.3) | 57 (14.5) | 73 (9.6) | 52 (10.4) | 264 (11.6) |
| Increased work hours | 93 (15.1) | 53 (13.5) | 122 (16.0) | 92 (18.4) | 360 (15.8) |
| Accessed government support4 | 207 (33.6) | 151 (38.4) | 266 (34.9) | 171 (34.3) | 795 (35.0) |
| Educational attainment (children, N) | 608 | 394 | 796 | 489 | 2,287 |
| Homeschool | 3 (0.5) | 12 (3.0) | 21 (2.6) | 10 (2.0) | 46 (2.0) |
| Elementary | 514 (84.5) | 327 (83.0) | 576 (72.4) | 423 (86.5) | 1,840 (80.4) |
| Junior high | 18 (3.0) | 32 (8.1) | 101 (12.7) | 19 (3.9) | 170 (7.4) |
| High school | 18 (3.0) | 3 (0.8) | 27 (3.4) | 7 (1.4) | 55 (2.4) |
| Not in school | 55 (9.0) | 20 (5.1) | 71 (8.9) | 30 (6.1) | 176 (7.7) |
| Pandemic impact on school (children in school, N) | 550 | 362 | 704 | 449 | 2,065 |
| School closed in March 2020 | 454 (82.5) | 303 (83.7) | 626 (88.9) | 358 (79.7) | 1,741 (84.3) |
| Online classes offered | 445 (98.0) | 290 (95.7) | 544 (86.9) | 319 (89.1) | 1,598 (91.8) |
| Child attended online - fully | 369 (82.9) | 273 (94.1) | 446 (82.0) | 277 (86.8) | 1,365 (85.4) |
| Child attended online - partially | 72 (16.2) | 13 (4.5) | 89 (16.4) | 41 (12.9) | 215 (13.5) |
| School reopened before Jul 2020 | 232 (51.1) | 18 (5.9) | 115 (18.4) | 34 (9.5) | 399 (22.9) |
Values are presented as number (%).
CHILD, Canadian Healthy Infant Longitudinal Development; COVID-19, coronavirus disease 2019.
1 Values do not add to 100% because participants could select multiple responses.
2 On leave, unemployed, retired, stay at home parent.
3 Healthcare, delivery worker, store worker, security, building maintenance.
4 Mortgage or lease/rent payment deferral, personal income support ([e.g., Canadian Emergency Response Benefit, Canadian Emergency Student Benefit], employment insurance, business income support [e.g., Canadian Emergency Wage Subsidy], food bank).
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506.ArticlePubMedPMC
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239-1242.ArticlePubMed
- 3. Udell JA, Behrouzi B, Sivaswamy A, Chu A, Ferreira-Legere LE, Fang J, et al. Clinical risk, sociodemographic factors, and SARS-CoV-2 infection over time in Ontario, Canada. Sci Rep 2022;12:10534.ArticlePubMedPMCPDF
- 4. Galvão MH, Roncalli AG. Factors associated with increased risk of death from covid-19: a survival analysis based on confirmed cases. Rev Bras Epidemiol 2021;23:e200106.PubMed
- 5. Centers for Disease Control and Prevention. People with certain medical conditions. [cited 2022 May 16]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
- 6. Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among Black patients and White patients with COVID-19. N Engl J Med 2020;382:2534-2543.ArticlePubMed
- 7. Morales DR, Conover MM, You SC, Pratt N, Kostka K, DuarteSalles T, et al. Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. Lancet Digit Health 2021;3:e98-e114.ArticlePubMed
- 8. Smith C, Odd D, Harwood R, Ward J, Linney M, Clark M, et al. Deaths in children and young people in England after SARS-CoV-2 infection during the first pandemic year. Nat Med 2022;28:185-192.ArticlePubMedPDF
- 9. Sinaei R, Pezeshki S, Parvaresh S, Sinaei R. Why COVID-19 is less frequent and severe in children: a narrative review. World J Pediatr 2021;17:10-20.ArticlePubMedPDF
- 10. Syangtan G, Bista S, Dawadi P, Rayamajhee B, Shrestha LB, Tuladhar R, et al. Asymptomatic SARS-CoV-2 carriers: a systematic review and meta-analysis. Front Public Health 2021;8:587374.ArticlePubMedPMC
- 11. Weiner DL, Balasubramaniam V, Shah SI, Javier JR; Pediatric Policy Council. COVID-19 impact on research, lessons learned from COVID-19 research, implications for pediatric research. Pediatr Res 2020;88:148-150.ArticlePubMedPDF
- 12. Haug N, Geyrhofer L, Londei A, Dervic E, Desvars-Larrive A, Loreto V, et al. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat Hum Behav 2020;4:1303-1312.ArticlePubMedPDF
- 13. Subbarao P, Anand SS, Becker AB, Befus AD, Brauer M, Brook JR, et al. The Canadian Healthy Infant Longitudinal Development (CHILD) Study: examining developmental origins of allergy and asthma. Thorax 2015;70:998-1000.ArticlePubMed
- 14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-381.ArticlePubMed
- 15. Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208.ArticlePubMedPMC
- 16. Cholette F, Mesa C, Harris A, Ellis H, Cachero K, Lacap P, et al. Dried blood spot specimens for SARS-CoV-2 antibody testing: a multi-site, multi-assay comparison. PLoS One 2021;16:e0261003.ArticlePubMedPMC
- 17. World Health Organization. COVID-19 symptoms and severity; 2021 [cited 2022 Feb 15]. Available from: https://www.who.int/westernpacific/emergencies/covid-19/information/asymptomatic-covid-19.
REFERENCES
Figure & Data
References
Citations


 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite





