Articles
- Page Path
- HOME > Epidemiol Health > Volume 40; 2018 > Article
-
Cohort Profile
Korea HIV/AIDS Cohort Study: study design and baseline characteristics -
Bo Youl Choi1
 , Jun Yong Choi2
, Jun Yong Choi2 , Sang Hoon Han2
, Sang Hoon Han2 , Sang Il Kim3
, Sang Il Kim3 , Mee-Kyung Kee4
, Mee-Kyung Kee4 , Min Ja Kim5
, Min Ja Kim5 , Shin-Woo Kim6
, Shin-Woo Kim6 , Sung Soon Kim7
, Sung Soon Kim7 , Yu-Mi Kim8
, Yu-Mi Kim8 , Nam Su Ku2
, Nam Su Ku2 , Jin-Soo Lee9
, Jin-Soo Lee9 , Joo-Shil Lee10, Yunsu Choi1
, Joo-Shil Lee10, Yunsu Choi1 , Kyong Sil Park1,11
, Kyong Sil Park1,11 , Joon Young Song12
, Joon Young Song12 , Jun Hee Woo13
, Jun Hee Woo13 , Moon Won Kang3
, Moon Won Kang3 , June Kim2
, June Kim2
-
Epidemiol Health 2018;40:e2018023.
DOI: https://doi.org/10.4178/epih.e2018023
Published online: June 6, 2018
1Department of Preventive Medicine, Hanyang university College of Medicine, Seoul, Korea
2Department of Internal Medicine and AIDS Research Institute, Yonsei University College of Medicine, Seoul, Korea
3Division of Infectious Disease, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
4Division of Viral Disease Research, Center for Infectious Diseases Research, Korea, National Institute of Health, Cheongju, Korea
5Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
6Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea
7Center for Infectious Diseases Research, Korea National Institute of Health, Cheongju, Korea
8Department of Preventive Medicine, Dong-A University College of Medicine, Busan, Korea
9Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea
10Center for Immunology and Pathology, Cheongju, Korea
11Department of Nursing, Hanyang University School of Nursing, Seoul, Korea
12Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
13Department of Infectious Diseases, University of Ulsan College of Medicine, Seoul, Korea
- Correspondence: June Kim Department of Internal Medicine, Yonsei University College of Medicine, 50-1 Yonsei-ro, Seodaemun-gu, Seoul 03722, Korea E-mail: jmkim@yuhs.ac
©2018, Korean Society of Epidemiology
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- The number of persons infected by HIV/AIDS has consistently increased in Korea since the first case of HIV/AIDS infection in 1985 and reached 15,208 by 2016. About 1,100 new patients with HIV/ AIDS infections have emerged every year since 2013. In Korea, the Korea HIV/AIDS Cohort Study was established for the evidenced-based prevention, treatment, and effective management of patients infected with human immunodeficiency virus (HIV) in December 2006. This study monitored 1,438 patients, who accounted for about 10% of all patients with HIV/AIDS in Korea, for 10 years with the following aims: (1) to develop an administrative system for the establishment of a HIV/AIDS cohort-based study; (2) to standardize methodologies and the case report forms; and (3) to standardize multi-cohort data and develop a data cleaning method. This study aims to monitor at least 1,000 patients (excluding those for whom investigation had been completed) per year (estimated number of patients who can be monitored by January 2018: 939). By December 2016, the sex distribution was 93.3% for men, and 6.7% for women (gender ratio, 13.9:1.0), and 98.9% of all participants were Korean. More than 50.0% of the participants were confirmed as HIV positive after 2006. This study reports competitive, long-term research that aimed to develop policies for the prevention of chronic infectious diseases for patients with HIV. The data collected over the last decade will be used to develop indices for HIV treatment and health promotion.
- Since the recognition of acquired immune deficiency syndrome (AIDS), which gave rise to pneumocystis pneumonia and Kaposi’s sarcoma in young homosexual men across many cities, including Los Angeles, in the US in 1980 to 1981, the pathogenic organism have been isolated from patients with this syndrome and named lymphadenopathy-associated virus in 1983 [1-3]. Researchers named this immunodeficiency disorder as AIDS, which is caused by human immunodeficiency virus (HIV) [4-6].
- In the US, the Multicenter AIDS Cohort study was conducted on high-risk heterosexual and homosexual men in 1983, to understand the disease progression from HIV infection to AIDS expression and death [7]. The Amsterdam cohort study of the Netherlands [8], and the Swiss HIV cohort study of Switzerland were subsequently performed in 1984 and 1988, respectively [9]. The results of HIV/AIDS cohort studies from around the world, which were established in the early period after the recognition of AIDS, showed that the route of infection, immunological characteristics, characteristics of opportunistic infections, cause of death, and AIDS pathogenesis vary with country and race [10-12]. In 2003, Brazil established the HIV-Brazil Cohort and has been monitoring patients with HIV across 26 health facilities [13].
- According to the recent data released by the Joint United Nations Programme on HIV/AIDS, there were about 36.7 million adult survivors of HIV/AIDS, 1.8 million new persons infected by HIV/ AIDS, and one million deaths associated with AIDS in 2016 [14].
- In Korea, since the first report of HIV-positive patients (one Korean, and one foreigner) in 1985, the cumulative number of patients with HIV has increased to 15,208 (13,584 Koreans, and 1,624 foreigners) by 2016, over the last three decades. Of the 13,584 Koreans infected, 12,606 (92.8%) were men and 978 (7.2%) were women; the ratio of HIV-infected men is markedly higher. The cumulative mortality rate is 15.8%, with 2,134 mortalities of the 13,584 patients, 11,439 survivors of HIV/AIDS in 2016.
- Although the number of newly infected patients has been decreasing, the number of HIV-infected patients has increased 4 times since 2000 in Korea, and over 1,000 newly infected patients have emerged every year since 2013 [14,15]. Most domestic studies on HIV/AIDS have been conducted on patients from specific hospitals and have focused on assessing treatment effectiveness [16,17].
- The goal of this study was to understand the natural progression from AIDS onset until death, in the early period of HIV infection in Korean patients with HIV/AIDS who exhibit different epidemiological characteristics from foreigners. Additionally, to develop a management and treatment guideline for HIV/AIDS by investigating the epidemiological and clinical characteristics of these patients along with the identifying the factors that influence these characteristics. We started with the Korea HIV/AIDS Cohort Study (KoCosHIV), in which 15 medical institutions that have been treating patients with HIV/AIDS across the country since 2006 participated. A cohort of 1,438 patients has been established, and the patients were repeatedly surveyed.
INTRODUCTION
- Participating hospitals
- A total of 21 hospitals participated in the KoCosHIV, from December 2006 to December 2016. Of these, 15 mid-, and largescale general hospitals currently operate across six cities (2018).
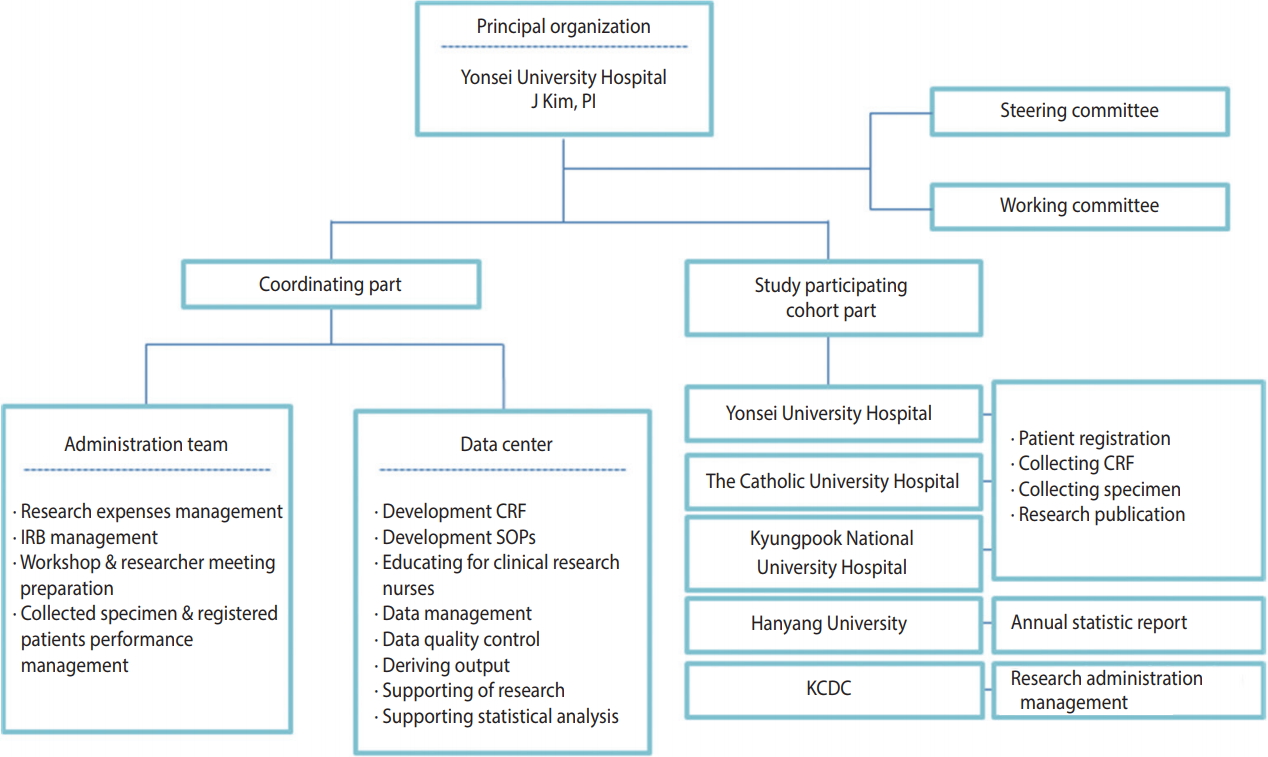
- This study was conducted by a research director, researchers from three research centers, an epidemiological team, and the department of viral diseases of the Korea National Institute of Health. The research director was responsible for the administrative tasks related to the research including conducting research, obtaining institutional review board (IRB) approval, and collecting data. The epidemiology research team was responsible for tasks related to data utilization, such as developing a standardized survey questionnaire and guideline, data cleansing, epidemiological consulting, providing and conducting statistical analyses. The Korea National Institute of Health has the rights to manage the consent form and use data to keep track of the yearly research progress, assign cohort management numbers, conduct participantbased repeated investigation, and manage biological resource samples (Figure 1). The administrative/clinical practice committee regularly met to share opinions related to the research and revised/ improved research tools and indices to make effective progress.
- Subjects
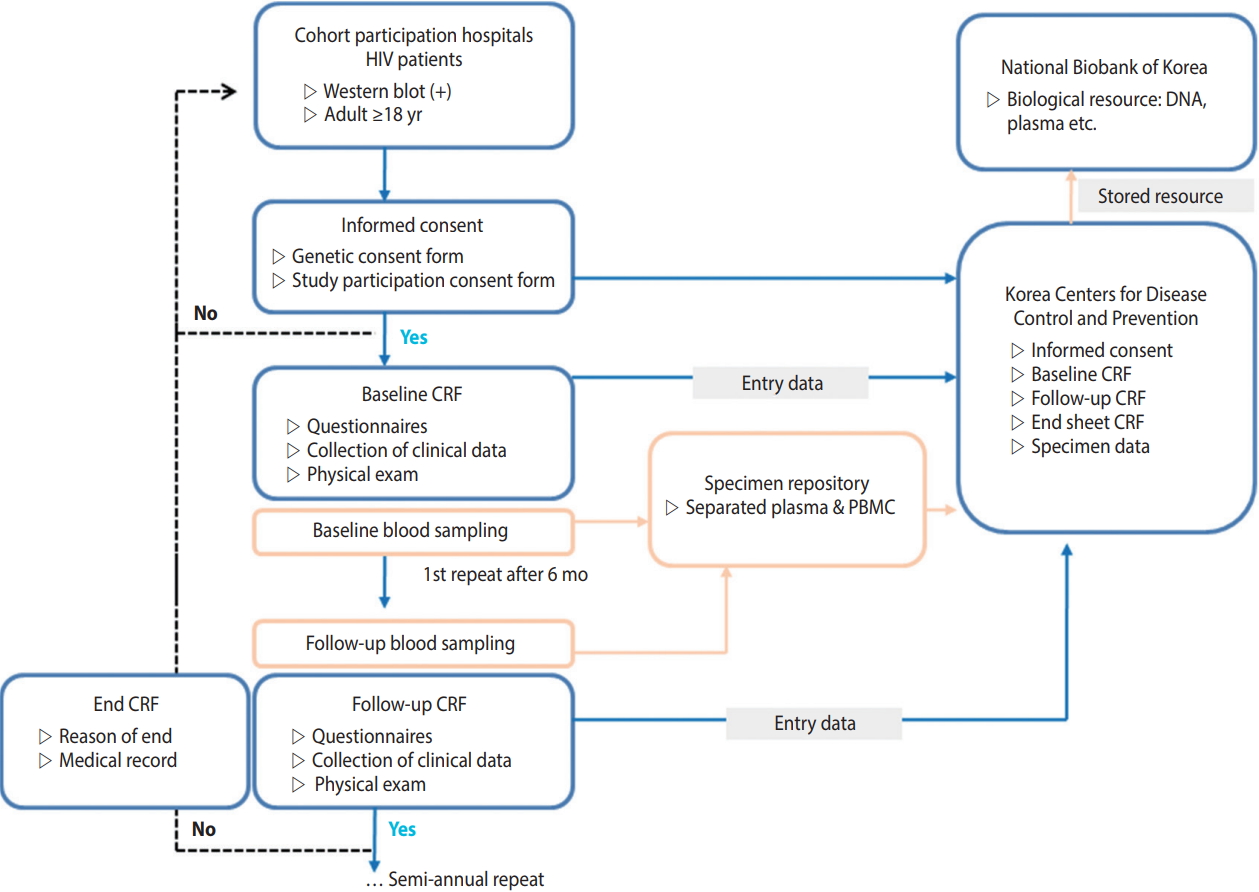
- HIV-infected Korean adults aged 18 years or older, who were confirmed as HIV positive by HIV Western blot, registered at the Korea Centers for Disease Control and Prevention (KCDC), had previous treatment experience at a participating hospital, and voluntarily consented to participating in this study after receiving a sufficient explanation of the research content, were included. This study is a cohort study, in which the dates of registration and research termination vary among the participants. Data were collected in real time from multiple centers by the Integrative Management System of the KCDC. The optimal date for the repeated investigation was six months after the investigation at the time of cohort registration. However, for participant convenience, a repeated investigation period was added from one to two months after the optimal date. Participants who were not surveyed for over two years after cohort registration were defined as “follow-up loss”. Reasons for ceasing participation, such as death or consented withdrawal, were documented in accordance with the survey format (Figure 2).
- Four men were registered by December 2006, and the number of HIV-infected participants consistently increased and reached 1,438 by 2016. Of the 13,584 HIV-infected Koreans in 2016, 13,152 were adults aged 20 years or older. Therefore, about 11% of the HIV-infected patients who were eligible for registration participated in this study (Table 1). Regarding the age distribution, there were 432 HIV-infected Koreans who were less than 20 years of age in 2016, and 11 of these patients participated in this study. About 10% of all infected Koreans between the ages of 20-40 years and 13% of all infected Koreans who were 40 years of age or older participated in this study (Table 2).
- Of the 1,438 participants who registered by December 31, 2016, over 50% were diagnosed as HIV positive after 2006. The mean age at cohort registration was 41.5 years, and the mean age at the time of diagnosis was 38.3 years. Of the Korean participants, 1.1% were naturalized foreigners. The immune status at the time of registration was determined by the number of CD4+ T cells and HIV RNA. The rates at which these two parameters were measured were 82.1 and 78.6%, respectively. There were 261 patients (18.2%) in the immunodeficiency group, with CD4+ T cell numbers less than 200, and 27 patients (1.9%) with HIV RNA numbers of 500,000 or greater (Table 3). According to national reports, only 6,192 Koreans (45.6%) underwent the CD4+ T cell test at the time of the report, of whom 2,387 (38.5% of all participants, or 17.6% of all HIV-infected Koreans) were in the immunodeficiency group (CD4< 200) [15].
- Data management
- The epidemiology research team manages data in three stages, to assure the data quality. First, the team educates clinical research nurses on standardized guidelines, before data collection. Second, the team performs real-time monitoring and manages the database to minimize errors that can occur during the data collection process. Third, the team statistically reviews the limiting values, outliers, and missing value of data, and develops an algorithm for logical error derivation that can occur from a question or between different investigation timings. Next, the team cleanses the data twice, to estimate errors, and confirms the results with the corresponding hospital. Furthermore, it provides a code book and a guideline on how to use the primitive data, to allow researchers to effectively use the cleansed data, and provides epidemiological consultation or statistical analysis when necessary.
- Research ethics
- All participating hospitals give consent for patient participation and provided test result information following IRB approval. Due to the nature of multi-year research projects, the study is continuously reviewed every year. To protect the patients’ personal information, each patient was assigned a cohort management number that did not include personal identification information, such as resident registration number, name, phone number, or address. When receiving data from the KCDC, the purpose of using the data and the date as well as destruction must be clearly stated and fulfilled. In addition, ethics education is regularly held for all researchers.
- Method of investigation
- The date of registration varied among the participants, and new participants were recruited every year. For patients for whom the basic investigation was completed, repeated investigations of treatment and disease were conducted every six months. For participants who did not show short-term changes, such as changes in marital statuses and health behaviors, the investigation was repeatedly conducted every 12 months. At least 1,000 participants were maintained every year, and research nurses performed quarterly updates on survey rates. For participants who could not be repeatedly surveyed due to withdrawal or hospital transfer, a follow-up investigation using nationally reported data was used once every year, to investigate whether or not the participants were deceased. By January 2018, a total of 939 participants could be followed-up (excluding those for whom research was terminated).
- The participants answered a questionnaire that contained questions about basic information, health behaviors (smoking/alcohol use), route of infection, sociopsychological state (depression, anxiety, quality of life), family history, medical history, vaccination history, and symptoms related to HIV/AIDS during the basic investigation period. Medical history, vaccination history, and recent symptoms related to HIV/AIDS were investigated every six months.
- Professional research nurses recorded patient treatment histories related to HIV/AIDS and associated opportunistic infections by referring to medical records and patient interviews, instead of using self-reported questionnaire surveys. The date of prescription and types of prescribed medications were recorded in detail. After the basic investigation period, all diseases that occurred after HIV diagnosis were recorded, in chronological order. In the repeated investigation, histories of diseases that occurred after the last investigation were recorded, and data were collected over time (Table 4).
- The participants were adults aged 18 years or older, with no significant predicted height changes, and height was assessed in the basic investigation only. However, parameters that were prone to changes such as weight, waist circumference, and hip circumference were measured every six months, in accordance with the guidelines. All body measurements were recorded up to the first decimal place.
- It was recommended to record parameters that were examined during chest X-rays, in addition to a radiologist’s comments, and regularly assess these parameters every six months. However, if this was not possible, the assessment must be performed at least once per year. In a diagnostic test that injects purified protein derivatives isolated from a Mycobacterium tuberculosis culture and examines the delayed hypersensitivity reaction caused by memory T cells, the diameter of the lower arm, vertical to the major axis, is measured in mm. If the result is positive, the investigation is not repeated; however, investigation is repeated every six months if the result is negative. The cervical pathological examination results were investigated and classified as normal or abnormal. Although the enzyme linked immuno-spot and QuantiFERON tests are not recommended for regular follow-up, they may be prescribed by the physician and performed every six months. Just like the purified protein derivatives of tuberculin skin test, they are not performed again if the result is positive and performed regularly if the result is negative. If a patient showed a response during the qualitative Treponema pallidum test, the quantified value was recorded. The standard unit for the immune test is 1 mm3 per number of cells, and the standard unit for viral load is copies/mL. In the patients for whom international units are used, these units must be converted to standard units. If no virus is detected, “0” was recorded. For the complete blood count test, only the test results were recorded using standard units. For hepatitis testing, if the qualitative test result was positive, we recommended to record quantitative test results as well, and the test was not repeated. If the result was negative, the test was performed regularly, every six months, at the discretion of the physician. Some parameters were assessed only during the basic investigation. For general chemical tests, the results of blood collections that were performed in the fasting state were recorded. The estimated glomerular filtration rate was automatically calculated, based on the registered values (gender, age, blood creatinine levels), using the isotope dilution mass spectrometry-modification of diet in renal disease equation (Table 4).
- It was recommended to collect biological resources at the time of each investigation. The institution providing the resources was responsible for shipping and producing biological resource (peripheral blood mononuclear cells, plasma) samples. Blood was collected into two cell preparation tubes (8 mL each) then shipped and processed on the same day as the collection. For hospitals in rural areas, under certain circumstances, samples were allowed to be shipped on the same day as the collection then processed the next day, under certain circumstances. For sample preservation, samples were regularly sent to the Korea Biobank of the KCDC and permanently stored (Figure 2).
MATERIALS AND METHODS
Repeated and follow-up investigations
Self-reported questionnaire, and examiner questionnaire
Body measurements and clinical examinations
Obtaining biological resources
- A total of 1,483 participants participated in the basic investigation, between December 2006 and February 2018. Nine hundred and eighty-nine participants (66.6%) participated in four or more repeated investigations. The researchers published a total of nine papers in journals, including three on the therapeutic effect of highly active antiretroviral therapy and compliance [18-20], two on sociopsychological factors (anxiety, depression) [21,22], and four on HIV-related diseases, opportunistic infections, hepatitis, and metabolic disorders [23-26]. Several papers on the management of cohort data quality, epidemiological characteristics, route of infection, survival rates of HIV-infected patients and patients with AIDS, simultaneous diagnosis of tuberculosis, and characteristics of proton beam therapy have been written and are being submitted for publication in scholarly journals.
- Advantages/disadvantages
- Over 1,000 newly infected patients have emerged on an annual basis since 2013, and the age at infection has been decreasing, with 33.9% of newly infected patients in 2016 found to be in their 20’s [15]. This study is the only HIV/AIDS cohort study that has monitored the incidence of group 3 nationally notifiable communicable infections and the epidemiological and clinical data of patients with HIV/AIDS, requiring management measures collected over time. The data collected over the last 11 years until present (2018) may be used to establish a basis for successful research studies and effective policies for HIV-infected patients in Korea. In 2016, there were 4,004 HIV-infected patients in the 15 participating hospitals, which is equivalent to 29.5% of all Koreans with HIV infection. Thus, by encouraging consistent participation, data representativeness may be secured. In addition, these data can be used to study various topics since not only HIV/AIDS treatment, but also opportunistic infections, drug tolerance, and metabolic disorders were assessed in the early investigation.
- However, this study may contain selection bias caused by the lack of active participation by the participants, due to the negative stigma around HIV/AIDS and patients affected by HIV in Korea. The inherent limitations of multi-center cohort studies, and the fact that all participating hospitals were mid- and large-scale hospitals.
MAJOR FINDINGS
- Data were distributed only among the researchers who participated in this study, in accordance with the decision of the department of viral diseases of the KCDC, which is the main research institution as of March 2018. Data distribution for external research centers is currently being planned. Data can be used in future research, through a process that complies with cohort data distribution regulations.
DATA ACCESSIBILITY
ACKNOWLEDGEMENTS
SUPPLEMENTAL MATERIALS
| Enrollment year |
Korea HIV/AIDS Cohort Study |
Korea HIV/AIDS patients1 |
||||
|---|---|---|---|---|---|---|
| Men | Women | Cumulative n (%) | Men | Women | Cumulative n (%) | |
| 1985-2005 | 0 | 0 | 0 (0.0) | 3,427 | 355 | 3,827 (28.2) |
| 2006 | 4 | 0 | 4 (0.3) | 687 | 62 | 4,576 (33.7) |
| 2007 | 183 | 13 | 200 (13.9) | 698 | 42 | 5,316 (39.1) |
| 2008 | 260 | 19 | 479 (33.4) | 743 | 54 | 6,113 (45.0) |
| 2009 | 142 | 11 | 632 (44.1) | 710 | 58 | 6,881 (50.7) |
| 2010 | 220 | 18 | 870 (60.6) | 723 | 50 | 7,654 (56.3) |
| 2011 | 175 | 12 | 1,057 (73.5) | 827 | 61 | 8,542 (62.9) |
| 2012 | 110 | 9 | 1,176 (81.8) | 808 | 60 | 9,410 (69.3) |
| 2013 | 58 | 3 | 1,237 (86.0) | 946 | 67 | 10,423 (76.7) |
| 2014 | 93 | 1 | 1,331 (92.6) | 1,016 | 65 | 11,504 (84.7) |
| 2015 | 56 | 8 | 1,395 (97.0) | 974 | 44 | 12,522 (92.2) |
| 2016 | 40 | 3 | 1,438 (100.0) | 1,002 | 60 | 13,584 (100.0) |
1 From Cho et al. HIV/AIDS notifications in Korea, 2016 [15].
| Age (yr) | Korea HIV/AIDS Cohort Study | Korea HIV/AIDS patients1 | Ratio (%) |
|---|---|---|---|
| <20 | 11 (0.01) | 432 (0.03) | 3 |
| 20-29 | 267 (0.19) | 3,523 (0.26) | 8 |
| 30-39 | 389 (0.27) | 3,699 (0.27) | 11 |
| 40-49 | 389 (0.27) | 3,000 (0.22) | 13 |
| 50-59 | 251 (0.17) | 1,922 (0.14) | 13 |
| 60+ | 131 (0.09) | 1,008 (0.07) | 13 |
| Total | 1,438 (100.0) | 13,584 (100.0) | 11 |
| Characteristics | Total | Men | Women | p-value |
|---|---|---|---|---|
| Total | 1,438 (100.0) | 1,341 (93.3) | 97 (6.7) | |
| Age at enrollment (yr) | ||||
| Mean±SD | 41.5±12.5 | 41.2±12.5 | 45.4±13.1 | 0.0011 |
| Median (IQR) | 41 (32-50) | 41 (32-50) | 48 (34-55) | |
| Age at diagnosed HIV (yr) | 1,432/1,438 (99.6) | 1,335/1,341 (99.6) | 97/97 (100.0) | |
| Mean±SD | 38.3±12.5 | 38.0±12.4 | 41.8±13.9 | 0.011 |
| Median (IQR) | 37 (28-46) | 37 (28-46) | 41 (30-53) | |
| Area of origin | 1,437/1,438 (99.9) | 1,340/1,341 (99.9) | 97/97 (100.0) | |
| Korean | 1,423 (98.9) | 1,337 (99.7) | 86 (88.7) | <0.0012 |
| Foreigner | 14 (1.0) | 3 (0.2) | 11 (11.3) | |
| Year of HIV diagnosis | 1,432/1,438 (99.6) | 1,335/1,341 (99.6) | 97/97 (100.0) | |
| Prior to 1990 | 3 (0.2) | 3 (0.2) | 0 (0.0) | 0.902 |
| 1990-1999 | 76 (5.3) | 69 (5.2) | 7 (7.2) | |
| 2000-2005 | 392 (27.3) | 364 (27.1) | 28 (28.9) | |
| 2006-2010 | 644 (44.8) | 601 (44.8) | 43 (44.3) | |
| 2011-2012 | 153 (10.6) | 145 (10.8) | 8 (8.3) | |
| 2013-2016 | 164 (11.4) | 153 (11.4) | 11 (11.3) | |
| CD4 cell count at enrollment (cell/mm3) | 1,180/1,438 (82.1) | 1,099/1,341 (82.0) | 81/97 (83.5) | |
| Median (IQR) | 371 (219-532.5) | 370 (218-526.0) | 405 (244-594.0) | 0.261 |
| ≥500 | 341 (23.7) | 310 (23.1) | 31 (32.0) | |
| 350-499 | 289 (20.1) | 276 (20.6) | 13 (13.4) | 0.11 |
| 200-349 | 290 (20.2) | 268 (19.9) | 22 (22.7) | |
| <200 | 260 (18.1) | 245 (18.3) | 15 (15.5) | |
| Viral load at enrollment (copies/mL) | 1,130/1,438 (78.6) | 1,052/1,341 (78.4) | 78/97 (80.4) | |
| Median (IQR) | 75 (20-15,867) | 75 (20-16,000) | 75 (19-14,000) | 0.481 |
| >500,000 | 27 (1.9) | 27 (2.0) | 0 (0.0) | |
| 1,000-500,000 | 418 (29.1) | 388 (28.9) | 30 (30.9) | 0.362 |
| 400-1,000 | 46 (3.2) | 45 (3.4) | 1 (1.0) | |
| ≤400 | 639 (44.4) | 592 (44.1) | 47 (48.5) |
BCG, bacillus Calmette–Guérin; HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome; HAART, highly active antiretroviral therapy; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; CMV, cytomegalovirus; PPD, purified protein derivative; TBc, tuberculosis; WBC, white blood cell; Hb, hemoglobin; Hct, hematocrit; HBsAg, hepatitis B antigen; Anti-HBs, anti-hepatitis B surface antibody; Anti-HBc, anti-hepatitis B core antibody; Anti-HCV, anti-hepatitis C virus; Anti-HAV IgG, anti-hepatitis A virus antibody immunoglobulin G; Anti-Hbe, anti-hepatitis B e-antigen; HBeAg, hepatitis B e-antigen; HBV-DNA, hepatitis B virus DNA detection test; HCV-PCR, hepatitis C virus-polymerase chain reaction test; CMV IgG, cytomegalovirus immunoglobulin G test; CMV IgM, cytomegalovirus immunoglobulin M; CMV RT PCR, cytomegalovirus real time polymerase chain reaction test; CMV Ag, cytomegalovirus antigen; FBS, fasting blood sugar; Total-C, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; TG, triglyceride; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma(γ) glutamyl transferase; T-B, total bilirubin; BUN, blood urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate.
- 1. Nelson KE, Celentano DD. Human immunodeficiency virus infection and the acquired immune deficiency syndrome. In: Nelson KE, Williams CM, editors. Infectious disease epidemiology. 3rd. Burlington: Jones & Barlett Learning; 2014. p 651-722.
- 2. Montagnier L. Lymphadenopathy-associated virus: from molecular biology to pathogenicity. Ann Intern Med 1985;103:689-693.ArticlePubMed
- 3. Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). 1983. Science 1983;220:868-871.ArticlePubMed
- 4. Friedman AH, Freeman WR, Orellana J, Kraushar MF, Starr MB, Luntz MH. Cytomegalovirus retinitis and immunodeficiency in homosexual males. Lancet 1982;1:958.Article
- 5. Self PC, Filardo TW, Lancaster FW. Acquired immunodeficiency syndrome (AIDS) and the epidemic growth of its literature. Scientometrics 1989;17:49-60.Article
- 6. Centers for Disease Control and Prevention (CDC). Pneumocystis pneumonia--Los Angeles. 1981. MMWR Morb Mortal Wkly Rep 1996;45:729-733.PubMed
- 7. Detels R, Jacobson L, Margolick J, Martinez-Maza O, Muñoz A, Phair J, et al. The multicenter AIDS Cohort Study, 1983 to…. Public Health 2012;126:196-198.ArticlePubMed
- 8. Coutinho RA. The Amsterdam Cohort Studies on HIV infection and AIDS. J Acquir Immune Defic Syndr Hum Retrovirol 1998;17 Suppl 1:S4-S8.ArticlePubMed
- 9. Swiss HIV Cohort Study, Schoeni-Affolter F, Ledergerber B, Rickenbach M, Rudin C, Günthard HF, et al. Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 2010;39:1179-1189.ArticlePubMedPDF
- 10. Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet 1999;353:863-868.ArticlePubMed
- 11. Lichtenstein B, Laska MK, Clair JM. Chronic sorrow in the HIVpositive patient: issues of race, gender, and social support. AIDS Patient Care STDS 2002;16:27-38.ArticlePubMed
- 12. Egger M, May M, Chêne G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002;360:119-129.ArticlePubMed
- 13. Grangeiro A, Escuder MM, Cassanote AJ, Souza RA, Kalichman AO, Veloso VG, et al. The HIV-Brazil cohort study: design, methods and participant characteristics. PLoS One 2014;9:e95673.ArticlePubMedPMC
- 14. Joint United Nations Programme on HIV/AIDS. The HIV-Brazil cohort study: design, methods and participant characteristics. UNAIDS data 2017 [cited 2018 Jul 13]. Available from: http://www.unaids.org/sites/default/files/media_asset/20170720_Data_book_2017_en.pdf.
- 15. Choi JH, Kim S, Park P, Cho KS. HIV/AIDS notifications in Korea. 2016 [cited 2018 Jul 31]. Available from: http://www.cdc.go.kr/CDC/cms/content/mobile/90/75790_view.html (Korean).
- 16. Kim JM, Cho GJ, Hong SK, Chang KH, Chung JS, Choi YH, et al. Epidemiology and clinical features of HIV infection/AIDS in Korea. Yonsei Med J 2003;44:363-370.ArticlePubMed
- 17. Sung H, Jung YS, Kang MW, Bae IG, Chang HH, Woo JH, et al. High frequency of drug resistance mutations in human immunodeficiency virus type 1-infected Korean patients treated with HAART. AIDS Res Hum Retroviruses 2007;23:1223-1229.ArticlePubMed
- 18. Song JY, Lee JS, Jung HW, Choi HJ, Lee JS, Lee J, et al. Primary anti-retroviral resistance in treatment-naive HIV-infected patients: a Korean HIV/AIDS cohort study. Infect Chemother 2009;41:230-232.Article
- 19. Kim MJ, Chang HH, Kim SI, Kim YJ, Park DW, Kang C, et al. Trend of CD4+ cell counts at diagnosis and initiation of highly active antiretroviral therapy (HAART): Korea HIV/AIDS Cohort Study, 1992-2015. Infect Chemother 2017;49:101-108.ArticlePubMedPMC
- 20. Kim MJ, Lee SA, Chang HH, Kim MJ, Woo JH, Kim SI, et al. Causes of HIV drug non-adherence in Korea: Korea HIV/AIDS Cohort Study, 2006-2015. Infect Chemother 2017;49:213-218.ArticlePubMedPMC
- 21. Kee MK, Lee SY, Kim NY, Lee JS, Kim JM, Choi JY, et al. Anxiety and depressive symptoms among patients infected with human immunodeficiency virus in South Korea. AIDS Care 2015;27:1174-1182.ArticlePubMed
- 22. Park KS. Impact of depression and anxiety on cardiovascular risk using Framingham score among HIV positivie patients: an analysis of the Korea Cohort HIV/AIDS Study. [dissertation]. Seoul: Hanyang University; 2015. (Korean).
- 23. Kim YJ, Woo JH, Kim MJ, Park DW, Song JY, Kim SW, et al. Opportunistic diseases among HIV-infected patients: a multicenternationwide Korean HIV/AIDS cohort study, 2006 to 2013. Korean J Intern Med 2016;31:953-960.ArticlePubMedPMCPDF
- 24. Oh DH, Ahn JY, Kim SI, Kim MJ, Woo JH, Kim WJ, et al. Metabolic complications among Korean patients with HIV infection: the Korea HIV/AIDS Cohort Study. J Korean Med Sci 2017;32:1268-1274.ArticlePubMedPMC
- 25. Kim YC, Ahn JY, Kim JM, Kim YJ, Park DW, Yoon YK, et al. Human immunodeficiency virus (HIV) and hepatitis virus coinfection among HIV-infected Korean patients: the Korea HIV/AIDS Cohort Study. Infect Chemother 2017;49:268-274.ArticlePubMedPMC
- 26. Kim EJ, Ahn JY, Kim YJ, Wie SH, Park DW, Song JY, et al. The prevalence and risk factors of renal insufficiency among Korean HIV-infected patients: the Korea HIV/AIDS Cohort Study. Infect Chemother 2017;49:194-204.ArticlePubMedPMC
REFERENCES
Figure & Data
References
Citations

- Smoothed quantile residual life regression analysis with application to the Korea HIV/AIDS cohort study
Soo Min Kim, Yunsu Choi, Sangwook Kang, Korea HIV/AIDS cohort study
BMC Medical Research Methodology.2024;[Epub] CrossRef - Rate of and Risk Factors for Loss to Follow Up in HIV-Infected Patients in Korea: The Korea HIV/AIDS Cohort Study
Hye Seong, Yunsu Choi, Minjeong Kim, Jung Ho Kim, Joon Young Song, Shin-Woo Kim, Sang Il Kim, Youn Jeong Kim, Dae Won Park, Boyoung Park, Bo Youl Choi, Jun-Yong Choi
Infection & Chemotherapy.2023; 55(1): 69. CrossRef - Effect of characteristics on the clinical course at the initiation of treatment for human immunodeficiency virus infection using dimensionality reduction
Yunsu Choi, Bo Youl Choi, Sang Il Kim, Jungsoon Choi, Jieun Kim, Bo Young Park, Soo Min Kim, Shin-Woo Kim, Jun Yong Choi, Joon Young Song, Youn Jeong Kim, Hyo Youl Kim, Jin-Soo Lee, Jung Ho Kim, Yoon Hee Jun, Myungsun Lee, Jaehyun Seong
Scientific Reports.2023;[Epub] CrossRef - Attachment Insecurity and Stigma as Predictors of Depression and Anxiety in People Living With HIV
Kyungmin Kim, Seoyoung Jang, Hyo-Deog Rim, Shin-Woo Kim, Hyun-ha Chang, Jungmin Woo
Psychiatry Investigation.2023; 20(5): 418. CrossRef - Assessment of Disease Burden and Immunization Rates for Vaccine-Preventable Diseases in People Living with HIV: The Korea HIV/AIDS Cohort Study
Hye Seong, Yunsu Choi, Kyoung Hwan Ahn, Jun Yong Choi, Shin-Woo Kim, Sang Il Kim, Mee-Kyung Kee, Bo Youl Choi, Boyoung Park, Hak Jun Hyun, Jin Gu Yoon, Ji Yun Noh, Hee Jin Cheong, Woo Joo Kim, Joon Young Song
Infection & Chemotherapy.2023; 55(4): 441. CrossRef - Risk Factors Associated with Medication Adherence in HIV/AIDS Patients
Kyung Sun Oh, Jin-soo Lee, Euna Han
Korean Journal of Clinical Pharmacy.2023; 33(4): 254. CrossRef - Effect of single tablet regimen on prescription trends for treatment-naïve patients with HIV/AIDS in Korea
Kyung Sun Oh, Gi Hyeon Seo, Hee Kyoung Choi, Euna Han
Scientific Reports.2022;[Epub] CrossRef - Predictors Associated With HIV Status Non-Disclosure in Korea
Kyungmin Kim, Jungmin Woo
Journal of Korean Medical Science.2022;[Epub] CrossRef - Comparison of Three Cardiovascular Risk Scores among HIV-Infected Patients in Korea: The Korea HIV/AIDS Cohort Study
Ji Yun Bae, Soo Min Kim, Yunsu Choi, Jun Yong Choi, Sang Il Kim, Shin-Woo Kim, Bo Young Park, Bo Youl Choi, Hee Jung Choi
Infection & Chemotherapy.2022; 54(3): 409. CrossRef - The Incidence and Risk Factors of Renal Insufficiency among Korean HIV infected Patients: The Korea HIV/AIDS Cohort Study
Jun Hyoung Kim, Heeseon Jang, Jung Ho Kim, Joon Young Song, Shin-Woo Kim, Sang Il Kim, Bo Youl Choi, Jun Yong Choi
Infection & Chemotherapy.2022; 54(3): 534. CrossRef - Estimation of the Number of HIV Infections and Time to Diagnosis in the Korea
Eunyoung Lee, Jungmee Kim, Jin Yong Lee, Ji Hwan Bang
Journal of Korean Medical Science.2020;[Epub] CrossRef - Human Immunodeficiency Virus-Associated Gastrointestinal Kaposi's Sarcoma: A Case Report
Hee Joong Lim, So Hyun Park, Seung Joon Choi, Suyoung Park, Hee Young Lee, Jun Won Chung, Dong Hae Chung
Journal of the Korean Society of Radiology.2020; 81(5): 1260. CrossRef - Epidemiological characteristics of HIV infected Korean: Korea HIV/AIDS Cohort Study
Yunsu Choi, Bo Youl Choi, Soo Min Kim, Sang Il Kim, June Kim, Jun Young Choi, Shin-Woo Kim, Joon Young Song, Youn Jeong Kim, Dae Won Park, Hyo Youl Kim, Hee-Jung Choi, Mee-Kyung Kee, Young Hyun Shin, Myeongsu Yoo
Epidemiology and Health.2019; 41: e2019037. CrossRef - Comparison of Characteristics and Survival between Prospective and Retrospective Korea Human Immunodeficiency Virus/Acquired Immune Deficiency Syndrome Cohort Studies
Jun Hyoung Kim, Yunsu Choi, Joon Young Song, Shin-Woo Kim, Sang Il Kim, Mee-Kyung Kee, Bo Youl Choi, Jun Yong Choi
Infection & Chemotherapy.2019; 51(4): 393. CrossRef

 KSE
KSE
 PubReader
PubReader ePub Link
ePub Link Cite
Cite



